Professional Documents
Culture Documents
Pharmacology Ms. Meenu Bhati (Asst. Prof at CBS) Adverse Drug Reactions
Pharmacology Ms. Meenu Bhati (Asst. Prof at CBS) Adverse Drug Reactions
Uploaded by
DAMBALE0 ratings0% found this document useful (0 votes)
53 views2 pagesThe document discusses different types of adverse drug reactions:
Type A reactions can be predicted from a drug's pharmacology and are dose-dependent. Type B reactions cannot be predicted and are not dose-dependent. Type C reactions can be predicted based on a drug or metabolite's chemical structure. Type D reactions occur after long-term treatment and can result from drug accumulation. Type E reactions occur when treatment ends, especially abruptly. Each type is illustrated with examples of common adverse reactions.
Original Description:
Hgf
Original Title
ADR (1)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses different types of adverse drug reactions:
Type A reactions can be predicted from a drug's pharmacology and are dose-dependent. Type B reactions cannot be predicted and are not dose-dependent. Type C reactions can be predicted based on a drug or metabolite's chemical structure. Type D reactions occur after long-term treatment and can result from drug accumulation. Type E reactions occur when treatment ends, especially abruptly. Each type is illustrated with examples of common adverse reactions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
53 views2 pagesPharmacology Ms. Meenu Bhati (Asst. Prof at CBS) Adverse Drug Reactions
Pharmacology Ms. Meenu Bhati (Asst. Prof at CBS) Adverse Drug Reactions
Uploaded by
DAMBALEThe document discusses different types of adverse drug reactions:
Type A reactions can be predicted from a drug's pharmacology and are dose-dependent. Type B reactions cannot be predicted and are not dose-dependent. Type C reactions can be predicted based on a drug or metabolite's chemical structure. Type D reactions occur after long-term treatment and can result from drug accumulation. Type E reactions occur when treatment ends, especially abruptly. Each type is illustrated with examples of common adverse reactions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Pharmacology Ms. Meenu Bhati (Asst.
Prof at CBS)
ADVERSE DRUG REACTIONS
Any noxious change which is suspected to be due to a drug, occurs at doses normally
used in man, requires treatment or decrease in dose or indicates caution in future use
of the same drug.
Adverse: Untoward, unintended, possibly causing harm (noxious)
Classification of ADRs:
Type A (Augmented)
Type B (Bizarre)
Type C (Chemical)
Type D (Delayed)
Type E (Exit/End of treatment)
1. Type A (Augmented) reactions:
Reactions which can be predicted from the known pharmacology of the drug,
Dose dependent,
Can be alleviated by a dose reduction.
E.g. Anticoagulants→Bleeding,
Prazosin→Postural hypotension………...
2. Type B (Bizarre) reactions:
Cannot be predicted from the pharmacology of the drug
Not dose dependent
E.g. Penicillin→Anaphylaxis
3. Type C (Chemical) reactions:
Biological characteristics can be predicted from the chemical structure of
the drug/metabolite
E.g. Paracetamol→Hepatotoxicity
4. Type D (Delayed) reactions:
Occur after many years of treatment.
Can be due to accumulation.
Pharmacology Ms. Meenu Bhati (Asst. Prof at CBS)
E.g. Chemotherapy→Secondary tumours
Phenytoin during pregnancy→Teratogenic effects
5. Type E (End of treatment) reactions:
Occur on withdrawal especially when drug is stopped abruptly.
E.g. Phenytoin withdrawal→Seizures
***END***
You might also like
- Test Bank For Pharmacotherapeutics For Advanced Practice 4th EditionDocument4 pagesTest Bank For Pharmacotherapeutics For Advanced Practice 4th EditionMichael Pupo59% (17)
- ADR Final1Document10 pagesADR Final1Rubina BisankheNo ratings yet
- Introduction Clinical Pharmacology 1Document228 pagesIntroduction Clinical Pharmacology 1gregparkianNo ratings yet
- Adr NewDocument43 pagesAdr Newshyamsundermaurya987No ratings yet
- Pharmacology Group 3Document9 pagesPharmacology Group 3wiyadiNo ratings yet
- AdrDocument22 pagesAdrM R RameshNo ratings yet
- General - Principle - of - Pharmacology 3Document7 pagesGeneral - Principle - of - Pharmacology 3Ntando MdhluliNo ratings yet
- Adverse Drug ReactionDocument5 pagesAdverse Drug Reactionfiena92No ratings yet
- Adverse Drug ReactionsDocument64 pagesAdverse Drug ReactionsSunanda mohanNo ratings yet
- Adverse Drug ReactionDocument24 pagesAdverse Drug ReactionGopal pokhrelNo ratings yet
- Adverse Eeffects of Drugs and Their Management in PaediatricsDocument6 pagesAdverse Eeffects of Drugs and Their Management in PaediatricsRinchin ChhotenNo ratings yet
- Adverse Drug ReactionsDocument34 pagesAdverse Drug ReactionsYanaNo ratings yet
- ADR Notes KINJAL S. GAMITDocument13 pagesADR Notes KINJAL S. GAMITKinjal GamitNo ratings yet
- Assignment A. List Down The Processes in Drug Development and Explain EachDocument2 pagesAssignment A. List Down The Processes in Drug Development and Explain EachIsabel PeraltaNo ratings yet
- Adverse Eeffects of Drugs and Their Management in PaediatricsDocument6 pagesAdverse Eeffects of Drugs and Their Management in PaediatricsRinchin ChhotenNo ratings yet
- Adverse Drug Reactions NotesDocument4 pagesAdverse Drug Reactions NoteskarthikeyanpgtNo ratings yet
- Pharmacodynamics: BIOL 122 DR Hemant MehtaDocument13 pagesPharmacodynamics: BIOL 122 DR Hemant MehtaRavinder KaurNo ratings yet
- Terminology in PharmacologyDocument31 pagesTerminology in PharmacologySaminathan Kayarohanam100% (2)
- Pharmacovigilance (Clinical Pharmacy)Document29 pagesPharmacovigilance (Clinical Pharmacy)Ameliya RoseNo ratings yet
- Adverse Drug ReactionsDocument41 pagesAdverse Drug ReactionsPopi Sopiah100% (1)
- Adverse Drug ReactionDocument108 pagesAdverse Drug ReactionBinod Sah100% (2)
- A Presentation On Adr Due To AntibioitcsDocument18 pagesA Presentation On Adr Due To AntibioitcsAnta SharmaNo ratings yet
- Adverse Effects of DrugsDocument31 pagesAdverse Effects of DrugsGANESH KUMAR JELLANo ratings yet
- Pharmacology Group 4Document12 pagesPharmacology Group 4mutiaraNo ratings yet
- Adrs 150226194556 Conversion Gate02Document56 pagesAdrs 150226194556 Conversion Gate02Ayu WijiNo ratings yet
- Adverse Drug ReactionsDocument16 pagesAdverse Drug ReactionsRJ MBBSNo ratings yet
- Classification of ADRDocument13 pagesClassification of ADRsai prasadNo ratings yet
- Review On Adverse Drug Reactions 2167 1052.1000005 RDocument2 pagesReview On Adverse Drug Reactions 2167 1052.1000005 RSourabh kundaraNo ratings yet
- Adverse Drug Reactions: Sudhakar LakavathDocument41 pagesAdverse Drug Reactions: Sudhakar LakavathSudhakar LakavathNo ratings yet
- Adverse Drug ReactionsDocument8 pagesAdverse Drug Reactionsapi-3781058No ratings yet
- 8.adverse Effects of DrugsDocument11 pages8.adverse Effects of DrugsOsama KhanNo ratings yet
- Adverse Drug ReactionDocument6 pagesAdverse Drug Reactionpranal patil (Pranal)No ratings yet
- ADRs - Classification, Mechanism, Predisposing Factors & Causality AssessmentDocument7 pagesADRs - Classification, Mechanism, Predisposing Factors & Causality AssessmentAman UpadhyayNo ratings yet
- Medications Error and ADRDocument3 pagesMedications Error and ADRالبتول تركستانيNo ratings yet
- Tinjauan Pustaka: Adverse Drug ReactionDocument9 pagesTinjauan Pustaka: Adverse Drug Reactionzulfaningsih HSNo ratings yet
- Adverse Drug EffectsDocument66 pagesAdverse Drug EffectsSuba Ranjana BalaNo ratings yet
- Pharm All NotesDocument511 pagesPharm All NotesShoaib Ali100% (1)
- Adverse Drug Reactions ADR Classes ADR Reporting ADRDocument37 pagesAdverse Drug Reactions ADR Classes ADR Reporting ADRMahum SohailNo ratings yet
- PT718Document15 pagesPT718Susmita GhoshNo ratings yet
- ADR Reaction, Medication Error and Drug InteractionDocument86 pagesADR Reaction, Medication Error and Drug Interactionada atienzaNo ratings yet
- Adverse Drug ReactionDocument6 pagesAdverse Drug ReactionNunkoo RajNo ratings yet
- ADR FinalDocument30 pagesADR FinalHarsh BhardwajNo ratings yet
- ADVERSE DRUG REACTIONS - PharmaDocument53 pagesADVERSE DRUG REACTIONS - PharmaShreepriya KhuranaNo ratings yet
- The Principles of Drugs Prescribing & Drug InteractionDocument87 pagesThe Principles of Drugs Prescribing & Drug InteractionRastia AlimmattabrinaNo ratings yet
- Adverse Reaction To DrugsDocument25 pagesAdverse Reaction To Drugsbv2328002No ratings yet
- Drug InteractionDocument24 pagesDrug InteractionChuol Mateat KanNo ratings yet
- Adverse Drug Reactions: Towards Mechanism-Based and Translational Strategies Nico P. E. VermeulenDocument2 pagesAdverse Drug Reactions: Towards Mechanism-Based and Translational Strategies Nico P. E. Vermeulenmegazhang94No ratings yet
- Adverse Effects of Drug Therapy: Cured Yesterday of My Disease, I Died Last Night of My PhysicianDocument10 pagesAdverse Effects of Drug Therapy: Cured Yesterday of My Disease, I Died Last Night of My PhysicianRoderick RichardNo ratings yet
- Unit 1Document15 pagesUnit 1kunalNo ratings yet
- ADR@SAEDocument23 pagesADR@SAEapi-3810976No ratings yet
- ADRs, Reporting and Causality AssessmentDocument37 pagesADRs, Reporting and Causality AssessmentRaymond ManjengwaNo ratings yet
- Drug Interactions FinalDocument27 pagesDrug Interactions FinalPrathyusha PandithaNo ratings yet
- Xamar CadeyDocument48 pagesXamar Cadeyamumahammed19No ratings yet
- Adverse Drug Reactions Definitions, Diagnosis, and ManagementDocument5 pagesAdverse Drug Reactions Definitions, Diagnosis, and Managementvinicius_barrosNo ratings yet
- Adverse Reactions - EDocument92 pagesAdverse Reactions - EYeshaa MiraniNo ratings yet
- Adverse Drug ReactionsDocument14 pagesAdverse Drug Reactionsgaikwaduv98No ratings yet
- Adr and ManagementDocument48 pagesAdr and ManagementDhanush G V DhanushNo ratings yet
- N-Lec3 - PharmacotherapeuticsDocument29 pagesN-Lec3 - Pharmacotherapeuticsgeng gengNo ratings yet
- Adverse Drug ReactionDocument3 pagesAdverse Drug ReactionJoan Espita Valencia-LealNo ratings yet
- PGY PR Attendance ReportDocument4 pagesPGY PR Attendance ReportDAMBALENo ratings yet
- Epilepsia - 2002 - Anderson - Children Versus Adults Pharmacokinetic and Adverse Effect DifferencesDocument7 pagesEpilepsia - 2002 - Anderson - Children Versus Adults Pharmacokinetic and Adverse Effect DifferencesDAMBALENo ratings yet
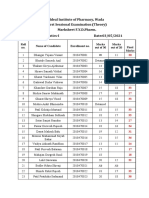
- Pgy PR Sessional-Iii MarksheetDocument3 pagesPgy PR Sessional-Iii MarksheetDAMBALENo ratings yet
- PC-I, FY 2nd Sessional (PR) ReportDocument13 pagesPC-I, FY 2nd Sessional (PR) ReportDAMBALENo ratings yet
- BLUEPRINT - Basic Principles of Toxicology Elective TYDocument2 pagesBLUEPRINT - Basic Principles of Toxicology Elective TYDAMBALENo ratings yet
- Tax Invoice: Shipping Address: Sold By: Invoice DetailsDocument1 pageTax Invoice: Shipping Address: Sold By: Invoice DetailsDAMBALENo ratings yet
- 8TH Sem Project MarksheetDocument5 pages8TH Sem Project MarksheetDAMBALENo ratings yet
- PH-I Sessional 2. Exam MARKSHEETDocument3 pagesPH-I Sessional 2. Exam MARKSHEETDAMBALENo ratings yet
- Blue Print PHARMACOLOGY-I (1) - 2Document1 pageBlue Print PHARMACOLOGY-I (1) - 2DAMBALENo ratings yet
- Ideal Institute of Pharmacy - Posheri - Wada B-Pharmacy Ii ND Year 2020-21Document12 pagesIdeal Institute of Pharmacy - Posheri - Wada B-Pharmacy Ii ND Year 2020-21DAMBALENo ratings yet
- Pradnya - Sheet1Document1 pagePradnya - Sheet1DAMBALENo ratings yet
- Pharmaceutics - I (Theory) Set-ADocument1 pagePharmaceutics - I (Theory) Set-ADAMBALENo ratings yet
- University of Mumbai: Fourth Year B. of Pharmacy (Sem VIII) (CBCS)Document1 pageUniversity of Mumbai: Fourth Year B. of Pharmacy (Sem VIII) (CBCS)DAMBALENo ratings yet
- BLUEPRINT - Basic Principles of Toxicology Elective TYDocument2 pagesBLUEPRINT - Basic Principles of Toxicology Elective TYDAMBALENo ratings yet
- Not Return Teachers Book ListDocument1 pageNot Return Teachers Book ListDAMBALENo ratings yet
- Discussions: Comparative Computational Study of Nipah Virus DrugsDocument21 pagesDiscussions: Comparative Computational Study of Nipah Virus DrugsDAMBALENo ratings yet
- Comparative Computational Study On Nipah Virus DrugsDocument10 pagesComparative Computational Study On Nipah Virus DrugsDAMBALENo ratings yet
- Website: WWW - Niperhyd.ac - in / WWW - Niperhyd.edu - In: TH THDocument24 pagesWebsite: WWW - Niperhyd.ac - in / WWW - Niperhyd.edu - In: TH THDAMBALENo ratings yet
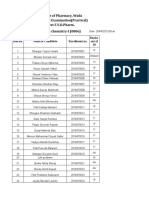
- SR - NO. Name of The Student Contact No. Contact No. Course DateDocument1 pageSR - NO. Name of The Student Contact No. Contact No. Course DateDAMBALENo ratings yet
- Comparativecomputational Study On Nipah Virus DrugsDocument10 pagesComparativecomputational Study On Nipah Virus DrugsDAMBALENo ratings yet
- Teaching AdvertisementDocument9 pagesTeaching AdvertisementDAMBALENo ratings yet
- Siddhi Bhagwan Dalvi ....Document5 pagesSiddhi Bhagwan Dalvi ....DAMBALENo ratings yet