Professional Documents
Culture Documents
CL O CL: 4. Green Chemistry in Day-to-Day Life
Uploaded by
Saurav PaulOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CL O CL: 4. Green Chemistry in Day-to-Day Life
Uploaded by
Saurav PaulCopyright:
Available Formats
4.
Green Chemistry in Day-to-Day Life
With the advancement of science, green chemistry has changed our life style.
Some of its important applications are described.
4.1 Dry Cleaning of Clothes
Perchloroethylene (PERC), CI 2C=CCI2 is commonly being used as a solvent
for dry cleaning. It is now known that PERC contaminates ground water and
is a suspected human carcinogen.
A technology, known as Micell Technology developed by Joseph De
Simons, Timothy Romack, and James McClain made use of liquid carbon
dioxide and a surfactant for dry cleaning clothes, thereby replacing PERC.
Dry cleaning machines have now been developed using this technique.
Micell technology has also evolved a metal-cleaning system that uses carbon
dioxide and a surfactant, thereby eliminating the need of halogenated solvents·.
4.2 Versatile Bleaching Agent
It is common knowledge that paper is manufactured from wood (which
contains about 70% polysaccharides and about 30% lignin). For good quality
paper, the lignin must be completely removed. Initially, lignin is removed by
placing small chipped pieces of wood into a bath of sodium hydroxide and
sodium sulfide (that is how pulp is formed). By this process about 80-90% of
lignin is decomposed. The remaining lignin was so far removed through reaction
with chlorine gas. The use of chlorine removes all the lignin (to give good
quality white paper) but causes environmental problems. Chlorine also reacts
with aromatic rings of the lignin (by aromatic substitution) to produce dioxins,
such as 2,3,7 ,8-tetrachloro-p-dioxin and chlorinated furans. These compounds
are potential carcinogens and cause other health problems.
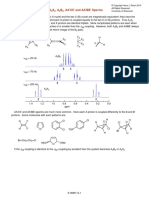
Cl
Cl~0YyCl ---i+-Cl
Cl~O~Cl
2,3,7,8-Tetrachloro- Chlorinated
dibenzo-p-dioxin furans
V. K. Ahluwalia et al., New Trends in Green Chemistry
© Anamaya Publishers, New Delhi, India 2004
16 GREEN CHEMISTRY
These halogenated products find their way into the food chain and finally
into products like dairy products, pork, beef and fish. In view of this, use of
chlorine has been discouraged. Subsequently, chlorine dioxide was used. Other
bleaching agents like hydrogen peroxide, ozone or oxygen also did not give the
desired results.
A versatile bleaching agent has been developed by Terrence Collins of
Carnegie Mellon University. It involves the use of hydrQgen peroxide as a
bleaching agent in the presence of some activators known as TAML activatorsz
that act as catalysts which promote the conversion of HzOz into hydroxyl
radicals that are involved in oxidationlbleaching. The catalytic activity ofTAML
activators allows HPz to break down more lignin in a shorter time and at
much lower temperature.
These bleaching agents also find use in laundry and result in lesser use of
water.
References
1. Miceli Technologies, Website: wwwmicell.com, accessed Dec. 1999.
2. J.A. Hall, L.D. Vuocolo, l.D. Suckling, C.P. HOIwitz, R.w. Allison, L.J. Wright and
T. Collins, A new catalysed hydrogen peroxide bleaching process, Proceedings of the
53,d APPITA Annual Conference April 19-22, 1999, Rotorua, New Zealand.
You might also like
- A Highly Selective and Sensitive Fluorescent Chemosensor For Fe in Physiological Aqueous SolutionDocument2 pagesA Highly Selective and Sensitive Fluorescent Chemosensor For Fe in Physiological Aqueous SolutionSaurav PaulNo ratings yet
- Optical studies reveal nature of "blue phaseDocument2 pagesOptical studies reveal nature of "blue phaseSaurav PaulNo ratings yet
- High Mobility Hole TransportDocument7 pagesHigh Mobility Hole TransportSaurav PaulNo ratings yet
- Voltagedependent Optical Activity of A Twisted Nematic Liquid CrystalDocument3 pagesVoltagedependent Optical Activity of A Twisted Nematic Liquid CrystalSaurav PaulNo ratings yet
- 7-Ion CHDocument3 pages7-Ion CHSaurav PaulNo ratings yet
- CV 1st PartDocument3 pagesCV 1st PartSaurav PaulNo ratings yet
- Firth BMJ1991 Chaos Predicting UnpredictableDocument4 pagesFirth BMJ1991 Chaos Predicting UnpredictableRakesh Babu100% (1)
- Designing A Green Synthesis: of StartingDocument3 pagesDesigning A Green Synthesis: of StartingSaurav PaulNo ratings yet
- Dielectric Materials: Dielectric Materials Are and Used Principally in andDocument7 pagesDielectric Materials: Dielectric Materials Are and Used Principally in andSaurav PaulNo ratings yet
- Scanned by CamscannerDocument2 pagesScanned by CamscannerSaurav PaulNo ratings yet
- SINTETIS Ibuprofen PDFDocument6 pagesSINTETIS Ibuprofen PDFRodolfo Angulo OlaisNo ratings yet
- Nakanishi 2004Document9 pagesNakanishi 2004Saurav PaulNo ratings yet
- Tetrahedron Letters: Hiromasa Mitsudera, Chao-Jun LiDocument3 pagesTetrahedron Letters: Hiromasa Mitsudera, Chao-Jun LiSaurav PaulNo ratings yet
- Structure Problem Solving Using H and C NMR Spectral Data Tutorial SessionDocument12 pagesStructure Problem Solving Using H and C NMR Spectral Data Tutorial SessionSaurav PaulNo ratings yet
- Ahluwalia 2004Document4 pagesAhluwalia 2004Saurav PaulNo ratings yet
- Factors Affecting Vibrational Frequenciesand IR Spectroscopy of HydrocarbonsDocument17 pagesFactors Affecting Vibrational Frequenciesand IR Spectroscopy of HydrocarbonsMuhammad HussnainNo ratings yet
- 13C NMR ConceptsDocument15 pages13C NMR ConceptsSaurav PaulNo ratings yet
- Ahluwalia 2004Document1 pageAhluwalia 2004Saurav PaulNo ratings yet
- 1455787242CHE P12 M30 EtextDocument8 pages1455787242CHE P12 M30 EtextSaurav PaulNo ratings yet
- 1455787242CHE P12 M30 EtextDocument8 pages1455787242CHE P12 M30 EtextSaurav PaulNo ratings yet
- Cross Dehydrogenative CouplingDocument10 pagesCross Dehydrogenative CouplingAnonymous rm2rf6No ratings yet
- Application of IR Spectroscopy and Interpretation of IR SpectrumDocument10 pagesApplication of IR Spectroscopy and Interpretation of IR SpectrumMuhammad HussnainNo ratings yet
- 1455786764CHE P12 M24 E-TextDocument10 pages1455786764CHE P12 M24 E-TextSaurav PaulNo ratings yet
- Applications of 13C NMR in Structural DeterminationDocument13 pagesApplications of 13C NMR in Structural DeterminationSaurav PaulNo ratings yet
- 1455877785CHE P12 M35 EtextDocument8 pages1455877785CHE P12 M35 EtextSaurav PaulNo ratings yet
- 1455787432CHE P12 M33 E-TextDocument12 pages1455787432CHE P12 M33 E-TextSaurav PaulNo ratings yet
- 1455787138CHE P12 M29 EtextDocument7 pages1455787138CHE P12 M29 EtextSaurav PaulNo ratings yet
- Account Statement From 1 Sep 2019 To 11 Aug 2020: TXN Date Value Date Description Ref No./Cheque No. Debit Credit BalanceDocument2 pagesAccount Statement From 1 Sep 2019 To 11 Aug 2020: TXN Date Value Date Description Ref No./Cheque No. Debit Credit BalanceSaurav PaulNo ratings yet
- AA'BB' SpectraDocument11 pagesAA'BB' SpectraSaurav PaulNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Quaternary Structure of ProteinDocument18 pagesQuaternary Structure of ProteinRoopa RanganathanNo ratings yet
- Armor X 3C XHHW-2 Power 3GND 600V 2017 - CCDocument2 pagesArmor X 3C XHHW-2 Power 3GND 600V 2017 - CCpglv0210No ratings yet
- Qdoc - Tips Practical Handbook of Grouting Soil Rock and Struc 19 43Document25 pagesQdoc - Tips Practical Handbook of Grouting Soil Rock and Struc 19 43Diego Quispe CondoriNo ratings yet
- Permit To Work (PTW) AwarenessDocument13 pagesPermit To Work (PTW) AwarenessNur FaizahNo ratings yet
- 1 s2.0 S095006181933380X AmDocument13 pages1 s2.0 S095006181933380X AmvajdazitaNo ratings yet
- Ionic Equilibrium Sheet-1 12.11.2021Document5 pagesIonic Equilibrium Sheet-1 12.11.2021sreevaishnava01No ratings yet
- Aakash Intensive CST 01-A (@neet - Nikalo1)Document39 pagesAakash Intensive CST 01-A (@neet - Nikalo1)all India TamilNo ratings yet
- Material Balance in Froth Flotation Using Microsoft Excel SolverDocument36 pagesMaterial Balance in Froth Flotation Using Microsoft Excel Solverjoseph kafumbila97% (62)
- IRJET-V5I8154-Response Spectrum Modelling Intze Tank PDFDocument6 pagesIRJET-V5I8154-Response Spectrum Modelling Intze Tank PDFRamkumar KumaresanNo ratings yet
- Assessment of Sugars Separation From A Model Carbohydrates Solution by Nanofiltration Using A Design of Experiments (DoE) MethodologyDocument10 pagesAssessment of Sugars Separation From A Model Carbohydrates Solution by Nanofiltration Using A Design of Experiments (DoE) MethodologyMaria AlvarezNo ratings yet
- CH 2 WaterDocument52 pagesCH 2 WaterahmedaznjadatNo ratings yet
- CHM457 - Exp 4-Preparation of Aspirin - N.syamimi BT Zainal - As2452s1 PDFDocument5 pagesCHM457 - Exp 4-Preparation of Aspirin - N.syamimi BT Zainal - As2452s1 PDFsyamimi zainalNo ratings yet
- Laboratory Simulation of Corosion Damage in Reinforced ConcreteDocument14 pagesLaboratory Simulation of Corosion Damage in Reinforced ConcretenitaainindiaNo ratings yet
- Chem 3Document14 pagesChem 3Ellaine NacisNo ratings yet
- CHE222 Important Course InformationDocument45 pagesCHE222 Important Course InformationStudent 365No ratings yet
- Chemistry SPM State Trial Papers-Form5chap1Document17 pagesChemistry SPM State Trial Papers-Form5chap1Law Jin YaoNo ratings yet
- Sticky Molecules - StudentDocument6 pagesSticky Molecules - StudentVanessa MurphyNo ratings yet
- The Iodometric Estimation of MercaptansDocument2 pagesThe Iodometric Estimation of MercaptansSteven Alvarez AguilarNo ratings yet
- Method of Test For Bulk Relative Density of Compacted Bituminous MixturesDocument3 pagesMethod of Test For Bulk Relative Density of Compacted Bituminous Mixturesming_zhu10No ratings yet
- Aking Osmetics: Certificate of AnalysisDocument1 pageAking Osmetics: Certificate of AnalysisAGATHA RIA BUDIYANANo ratings yet
- Periodic Table TrendsDocument1 pagePeriodic Table TrendsClick LinkNo ratings yet
- HT Ing Kyd 6110Document1 pageHT Ing Kyd 6110Guilherme PortellaNo ratings yet
- Test On Chemical KineticsDocument4 pagesTest On Chemical Kineticsdevansh dewanNo ratings yet
- Matrix Science Academy: Chemistry MHT CET L1 2022-23 Hints and SolutionsDocument2 pagesMatrix Science Academy: Chemistry MHT CET L1 2022-23 Hints and SolutionsLight MayNo ratings yet
- Amicon Ultra-15 Centrifugal Filter Devices: User GuideDocument6 pagesAmicon Ultra-15 Centrifugal Filter Devices: User GuideGus PolentaNo ratings yet
- L1 Measurement-StudentDocument66 pagesL1 Measurement-StudentNN JKNo ratings yet
- Rtu M Tech Thesis FormatDocument6 pagesRtu M Tech Thesis Formataprilscrantonspringfield100% (2)
- Synthesis and Characterization of Oligosalicylaldehyde-Based Epoxy ResinsDocument5 pagesSynthesis and Characterization of Oligosalicylaldehyde-Based Epoxy ResinsFrancesca TeocoliNo ratings yet
- Experiment 4 Stoichiometry and Theoretical YieldDocument8 pagesExperiment 4 Stoichiometry and Theoretical YieldFAtma HAnysNo ratings yet
- Unit 5 - Test Questions Humss 1 & Abm 3Document9 pagesUnit 5 - Test Questions Humss 1 & Abm 3Neil GabatoNo ratings yet