Professional Documents
Culture Documents
Bohrium: Borium Boron Barium
Uploaded by
RB KyrieOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Bohrium: Borium Boron Barium
Uploaded by
RB KyrieCopyright:
Available Formats
Bohrium
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Not to be confused with the chemical compound borium or
elements boron (B) or barium (Ba).
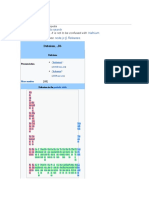
Bohrium, Bh 107
Bohrium
Pronunciation /ˈbɔːriəm/ ( listen) (BOR-ee-əm)
Mass number [270] (unconfirmed: 278)
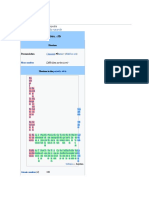
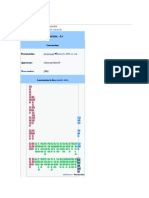
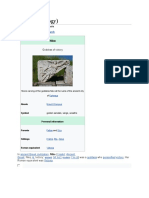
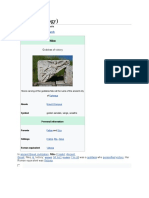
Bohrium in the periodic table
Hy
dro
ge
n
Lit Be
hiu ryll
m iu
m
So Ma
diu gn
m esi
um
Po Cal Sca
tas ciu ndi
siu m um
m
Ru Str Ytt
bid ont riu
iu iu m
m m
Ca Ba La C PrasNe Pro Sa Eu Ga Te Dy Ho Er ThuYt Lut
esi riu nth eri eod od me ma rop dol rbi spr lmi bi liu ter eti
um m an u ymi ym thi riu iu ini umosi um u m bi um
um m um iu um m m um um m u
m m
FraRa Ac T Prot Ur Ne Pl A Cu Be Cal Ein Fe Me NoLa
nci diu tiniho acti ani ptu ut me riu rkeiforstei rmnde belwr
um m um ri niu um niu on rici m liu niu niu iu levi iu enc
u m m iu um m m m m um m iu
m m m
seaborgium ←
Atomic number (Z) 107
Group group 7
Period period 7
Block d-block
Electron configuration [Rn] 5f14 6d5 7s2[1][2]
Electrons per shell 2, 8, 18, 32, 32, 13, 2
Physical properties
Phase at STP unknown phase (predicted)[3]
Density (near r.t.) 37.1 g/cm3 (predicted)[2][4]
Atomic properties
Oxidation states (+3), (+4), (+5), +7[2]
[4]
(parenthesized: prediction)
1st: 740 kJ/mol
Ionization energies
2nd: 1690 kJ/mol
3rd: 2570 kJ/mol
(more) (all but first estimated)
[2]
Atomic radius empirical: 128 pm (predicted)[2]
Covalent radius 141 pm (estimated)[5]
Other properties
Natural occurrence synthetic
Crystal structure hexagonal close-packed (hcp)
(predicted)[3]
CAS Number 54037-14-8
History
Naming after Niels Bohr
Discovery Gesellschaft für
Schwerionenforschung (1981)
Main isotopes of bohrium
Iso- Abun- Half-life Decay Pro-
tope dance (t1/2) mode duct
267
Bh syn 17 s α 263
Db
270
Bh syn 1 min α 266
Db
271
Bh syn 1.5 s
[6]
α 267
Db
272
Bh syn 11 s α 268
Db
274
Bh syn 44 s[7]
α 270
Db
278
Bh[8] syn 11.5 min? SF
Category: Bohrium
view
talk
edit
| references
Bohrium is a synthetic chemical element with the symbol Bh and atomic number 107. It
is named after Danish physicist Niels Bohr. As a synthetic element, it can be created in
a laboratory but is not found in nature. All known isotopes of bohrium are
extremely radioactive; the most stable known isotope is 270Bh with a half-life of
approximately 61 seconds, though the unconfirmed 278Bh may have a longer half-life of
about 690 seconds.
In the periodic table, it is a d-block transactinide element. It is a member of the 7th
period and belongs to the group 7 elements as the fifth member of the 6d series
of transition metals. Chemistry experiments have confirmed that bohrium behaves as
the heavier homologue to rhenium in group 7. The chemical properties of bohrium are
characterized only partly, but they compare well with the chemistry of the other group 7
elements.
You might also like
- Gravimetric Analysis: International Series of Monographs on Analytical Chemistry, Vol. 7From EverandGravimetric Analysis: International Series of Monographs on Analytical Chemistry, Vol. 7Rating: 2 out of 5 stars2/5 (1)
- Dubnium: Jump To Navigationjump To SearchDocument4 pagesDubnium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- Hassium: Jump To Navigationjump To SearchDocument4 pagesHassium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- Lawrencium: Jump To Navigationjump To SearchDocument4 pagesLawrencium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- This Article Is About The Element. For Other Uses, See - "Element 79" Redirects Here. For The Anthology, SeeDocument4 pagesThis Article Is About The Element. For Other Uses, See - "Element 79" Redirects Here. For The Anthology, SeeEllaineNo ratings yet
- CobaltDocument4 pagesCobaltIAN213No ratings yet
- Jump To Navigation Jump To Search: This Article Is About The Metallic Element. For Other Uses, SeeDocument52 pagesJump To Navigation Jump To Search: This Article Is About The Metallic Element. For Other Uses, SeeSAMUEL SANCHEZNo ratings yet
- Bismuth: Jump To Navigation Jump To SearchDocument21 pagesBismuth: Jump To Navigation Jump To SearchSAMUEL SANCHEZNo ratings yet
- Flerovium: Wiki Loves Monuments: Photograph A Monument, Help Wikipedia and Win!Document38 pagesFlerovium: Wiki Loves Monuments: Photograph A Monument, Help Wikipedia and Win!Jennie KimNo ratings yet
- Vimp Trends!!Document14 pagesVimp Trends!!Adarsh ThakareNo ratings yet
- This PDF Is The Sample PDF Taken From Our Comprehensive Study Material For IIT-JEE Main & AdvancedDocument13 pagesThis PDF Is The Sample PDF Taken From Our Comprehensive Study Material For IIT-JEE Main & AdvancedGod is every whereNo ratings yet
- SeaborgiumDocument8 pagesSeaborgiummikkasNo ratings yet
- Francium: Jump To Navigationjump To SearchDocument4 pagesFrancium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- EinstienniumDocument4 pagesEinstienniumIAN213No ratings yet
- Copper: Jump To Navigation Jump To SearchDocument32 pagesCopper: Jump To Navigation Jump To SearchSAMUEL SANCHEZNo ratings yet
- Jump To Navigation Jump To Search: This Article Is About The Chemical Element. For Other Uses, SeeDocument8 pagesJump To Navigation Jump To Search: This Article Is About The Chemical Element. For Other Uses, SeeRonaldNo ratings yet
- Actinium: Jump To Navigationjump To SearchDocument4 pagesActinium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- Nitrogen FamilyDocument11 pagesNitrogen FamilytldrsukeNo ratings yet
- Ruthenium: Jump To Navigation Jump To SearchDocument7 pagesRuthenium: Jump To Navigation Jump To SearchJianhengMokNo ratings yet
- Crystal Structure and Raman Spectral Studies of Baso - Pbso Solid SolutionDocument9 pagesCrystal Structure and Raman Spectral Studies of Baso - Pbso Solid SolutionintanNo ratings yet
- Boron FamilyDocument8 pagesBoron Familyomg667796saNo ratings yet
- Potassium NotesDocument33 pagesPotassium NotesTylerDargisNo ratings yet
- Azote: NitrogenDocument10 pagesAzote: NitrogenSerraji MaxNo ratings yet
- P-Block ElementsDocument14 pagesP-Block ElementsAviNo ratings yet
- Enplain Dfea: C I SeparlyDocument4 pagesEnplain Dfea: C I SeparlyAditya ChaturvediNo ratings yet
- D & F BlockDocument21 pagesD & F Blockyrajeshwari189No ratings yet
- Handbook of MetalDocument4 pagesHandbook of MetalLilian RoseNo ratings yet
- US6794333Document12 pagesUS6794333Amir RahbariNo ratings yet
- Rutherfordium: Rutherfordium Is A Synthetic ChemicalDocument46 pagesRutherfordium: Rutherfordium Is A Synthetic ChemicalAnonymous gUjimJKNo ratings yet
- Platinum: This Article Is About The Chemical Element. For Other Uses, SeeDocument4 pagesPlatinum: This Article Is About The Chemical Element. For Other Uses, SeeEllaineNo ratings yet
- Fermium: Jump To Navigationjump To SearchDocument3 pagesFermium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- (IA) Half-Heusler Alloy Possible or NotDocument12 pages(IA) Half-Heusler Alloy Possible or NotHoàng Thu ThuỷNo ratings yet
- Problem Set 2Document4 pagesProblem Set 2Kristine Joy PaltepNo ratings yet
- Maths 5 LessonDocument23 pagesMaths 5 Lessonrahot47884No ratings yet
- CBSE Class 12 Chemistry Solid State Practice Question SolutionsDocument15 pagesCBSE Class 12 Chemistry Solid State Practice Question SolutionsRiaNo ratings yet
- SeleniumDocument158 pagesSeleniumKishore KumarNo ratings yet
- JEE Main Online Exam 2019: Questions & Solutions (Memory Based)Document3 pagesJEE Main Online Exam 2019: Questions & Solutions (Memory Based)Ihtisham Ul HaqNo ratings yet
- CA Assignment 1Document6 pagesCA Assignment 1Daksh ChauhanNo ratings yet
- Nitrogen: Jump To Navigationjump To SearchDocument5 pagesNitrogen: Jump To Navigationjump To Searchelika.alfonsoNo ratings yet
- Americium: Jump To Navigationjump To SearchDocument5 pagesAmericium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- Periodic TableDocument137 pagesPeriodic TableIshfaqAhmedMayoNo ratings yet
- Periodic Properties - HandbookDocument12 pagesPeriodic Properties - HandbookHarsh KulkarniNo ratings yet
- 29 Apr 2022 ChemistryDocument23 pages29 Apr 2022 ChemistryAnantha PadmanabhanNo ratings yet
- Silver: This Article Is About The Chemical Element. For The Use of Silver As A Medication, See - For Other Uses, SeeDocument4 pagesSilver: This Article Is About The Chemical Element. For The Use of Silver As A Medication, See - For Other Uses, SeeEllaineNo ratings yet
- Nuclear Chemistry: Bit of A Problem SetDocument36 pagesNuclear Chemistry: Bit of A Problem SetStephanie Palomares LevitaNo ratings yet
- 6529242dd6ffef0018ccb77e - ## - P - Block (Part - 01)Document32 pages6529242dd6ffef0018ccb77e - ## - P - Block (Part - 01)justrohithingsNo ratings yet
- Copper: For Other Uses, SeeDocument4 pagesCopper: For Other Uses, SeeEllaineNo ratings yet
- Adobe Scan Feb 07, 2022Document7 pagesAdobe Scan Feb 07, 2022Soumya BawageNo ratings yet
- Tabla Correlaciones Analogía TDQ y TDMDocument1 pageTabla Correlaciones Analogía TDQ y TDMChristian PinoNo ratings yet
- Nitrogen, N: Jump To Navigation Jump To SearchDocument6 pagesNitrogen, N: Jump To Navigation Jump To SearchvenothNo ratings yet
- Neptunium: Jump To Navigationjump To SearchDocument4 pagesNeptunium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- Mod 2 AtcDocument6 pagesMod 2 AtcSathwik Rao KNo ratings yet
- CH 10. P-Block (Chem +1)Document48 pagesCH 10. P-Block (Chem +1)Sajag GargNo ratings yet
- Found: Boilina PeinDocument9 pagesFound: Boilina PeinVaibhav VyavahareNo ratings yet
- Davies 1973Document4 pagesDavies 1973Shu YanNo ratings yet
- Structural and Physical Properties of Rapidly Solidified Lead-Bismuth Eutectic AlloyDocument6 pagesStructural and Physical Properties of Rapidly Solidified Lead-Bismuth Eutectic AlloyRadu CristianNo ratings yet
- Lithium: Lithium (Medication) Lithium (Disambiguation)Document5 pagesLithium: Lithium (Medication) Lithium (Disambiguation)RB KyrieNo ratings yet
- Site Visit No.2Document12 pagesSite Visit No.2BritneyDNo ratings yet
- Oxygen Oxygen: Jump To Navigation Jump To SearchDocument46 pagesOxygen Oxygen: Jump To Navigation Jump To SearchSerraji MaxNo ratings yet
- Boron NitrideDocument18 pagesBoron NitrideFulgaNo ratings yet
- Hecate: Jump To Navigation Jump To SearchDocument4 pagesHecate: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Hypnos: Jump To Navigation Jump To SearchDocument2 pagesHypnos: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Hebe (Mythology) : Jump To Navigation Jump To SearchDocument3 pagesHebe (Mythology) : Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Tyche: Jump To Navigation Jump To SearchDocument3 pagesTyche: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Hecate: Jump To Navigation Jump To SearchDocument4 pagesHecate: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Hebe (Mythology) : Jump To Navigation Jump To SearchDocument3 pagesHebe (Mythology) : Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Nike (Mythology)Document2 pagesNike (Mythology)RB KyrieNo ratings yet
- Nike (Mythology) : Jump To Navigation Jump To SearchDocument2 pagesNike (Mythology) : Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Apollo: Jump To Navigation Jump To SearchDocument5 pagesApollo: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Jump To Navigation Jump To Search: For Other Uses, SeeDocument4 pagesJump To Navigation Jump To Search: For Other Uses, SeeRB KyrieNo ratings yet
- Hypnos: Jump To Navigation Jump To SearchDocument2 pagesHypnos: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Demeter: Jump To Navigation Jump To SearchDocument4 pagesDemeter: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Aeacus: Jump To Navigation Jump To SearchDocument1 pageAeacus: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Vance D. BrandDocument2 pagesVance D. BrandRB KyrieNo ratings yet
- Ilan Ramon: Jump To Navigation Jump To SearchDocument2 pagesIlan Ramon: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Michael S. Hopkins: Jump To Navigation Jump To SearchDocument2 pagesMichael S. Hopkins: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Victor J. Glover: Jump To Navigation Jump To SearchDocument2 pagesVictor J. Glover: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Christa McAuliffeDocument2 pagesChrista McAuliffeRB KyrieNo ratings yet
- Poseidon: Jump To Navigation Jump To SearchDocument4 pagesPoseidon: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Franklin Chang Díaz: Spanish Name SurnameDocument2 pagesFranklin Chang Díaz: Spanish Name SurnameRB KyrieNo ratings yet
- Bob Behnken: Jump To Navigation Jump To SearchDocument2 pagesBob Behnken: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Franklin Chang Díaz: Spanish Name SurnameDocument2 pagesFranklin Chang Díaz: Spanish Name SurnameRB KyrieNo ratings yet
- Doug Hurley: Jump To Navigation Jump To SearchDocument2 pagesDoug Hurley: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Jump To Navigation Jump To Search: For Other Uses, SeeDocument5 pagesJump To Navigation Jump To Search: For Other Uses, SeeRB KyrieNo ratings yet
- Demeter: Jump To Navigation Jump To SearchDocument4 pagesDemeter: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Americium: Jump To Navigationjump To SearchDocument5 pagesAmericium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- Hercules: Jump To Navigation Jump To SearchDocument2 pagesHercules: Jump To Navigation Jump To SearchRB KyrieNo ratings yet
- Jump To Navigation Jump To Search: For Other Uses, SeeDocument4 pagesJump To Navigation Jump To Search: For Other Uses, SeeRB KyrieNo ratings yet
- Francium: Jump To Navigationjump To SearchDocument4 pagesFrancium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- Neptunium: Jump To Navigationjump To SearchDocument4 pagesNeptunium: Jump To Navigationjump To SearchRB KyrieNo ratings yet
- LS3954A LS3954 LS3955 LS3956 LS3958: Linear Integrated SystemsDocument3 pagesLS3954A LS3954 LS3955 LS3956 LS3958: Linear Integrated SystemsJCMNo ratings yet
- Alpha Tech India Limited - FinalDocument4 pagesAlpha Tech India Limited - FinalRahul rNo ratings yet
- Consumer Price SummaryDocument5 pagesConsumer Price SummaryKJ HiramotoNo ratings yet
- Student's T DistributionDocument6 pagesStudent's T DistributionNur AliaNo ratings yet
- English 7 q3 Week2 Daily Lesson LogDocument5 pagesEnglish 7 q3 Week2 Daily Lesson LogKILVEN MASIONNo ratings yet
- Engineering Economics1Document64 pagesEngineering Economics1bala saiNo ratings yet
- Applied Social Research A Tool For The Human Services 9th Edition Monette Test Bank 1Document36 pagesApplied Social Research A Tool For The Human Services 9th Edition Monette Test Bank 1wesleyvasquezmeoapcjtrb100% (25)
- Topic 3 Module 2 Simple Annuity (Savings Annuity and Payout Annuity)Document8 pagesTopic 3 Module 2 Simple Annuity (Savings Annuity and Payout Annuity)millerNo ratings yet
- Med Error PaperDocument4 pagesMed Error Paperapi-314062228100% (1)
- Standards Guide 1021 1407Document8 pagesStandards Guide 1021 1407Anjur SiNo ratings yet
- Man of The House Faq: About MothDocument2 pagesMan of The House Faq: About MothPrapya BarmanNo ratings yet
- Provable Security - 8th International Conference, ProvSec 2014Document364 pagesProvable Security - 8th International Conference, ProvSec 2014alahbarNo ratings yet
- Oracle® Secure Backup: Installation and Configuration Guide Release 10.4Document178 pagesOracle® Secure Backup: Installation and Configuration Guide Release 10.4andrelmacedoNo ratings yet
- Ansi Numerical CodeDocument6 pagesAnsi Numerical Codekachra13No ratings yet
- Mineral Claim Purchase and Sale Agreement FinalDocument5 pagesMineral Claim Purchase and Sale Agreement Finaldaks4uNo ratings yet
- Real Estate (Regulation and Development) Act 2016 (RERA) CompliancesDocument15 pagesReal Estate (Regulation and Development) Act 2016 (RERA) CompliancesM S PrasadNo ratings yet
- Ref Drawing 2. Ref Code: 3. Design DatasDocument3 pagesRef Drawing 2. Ref Code: 3. Design DatasJoe Nadakkalan100% (3)
- Catalyst 4500 SeriesDocument1,230 pagesCatalyst 4500 SeriesnvleninkumarNo ratings yet
- 05271/MFP YPR SPL Sleeper Class (SL)Document2 pages05271/MFP YPR SPL Sleeper Class (SL)Rdx BoeNo ratings yet
- Methods of Data Collection MSC N I YrDocument256 pagesMethods of Data Collection MSC N I Yrdr.anu RkNo ratings yet
- Design and Implementation of Hotel Management SystemDocument36 pagesDesign and Implementation of Hotel Management Systemaziz primbetov100% (2)
- Karamadi - A Waning Practice of Shore Seine Operation Along Kerala, SouthwestDocument6 pagesKaramadi - A Waning Practice of Shore Seine Operation Along Kerala, SouthwestQ8123No ratings yet
- Antibiotic I and II HWDocument4 pagesAntibiotic I and II HWAsma AhmedNo ratings yet
- 033 - Flight Planning Monitoring - QuestionsDocument126 pages033 - Flight Planning Monitoring - QuestionsEASA ATPL Question Bank100% (4)
- ECDIS Presentation Library 4Document16 pagesECDIS Presentation Library 4Orlando QuevedoNo ratings yet
- AOCS Ca 12-55 - 2009 - Phosphorus PDFDocument2 pagesAOCS Ca 12-55 - 2009 - Phosphorus PDFGeorgianaNo ratings yet
- Skin Care Creams, Lotions and Gels For Cosmetic Use - SpecificationDocument33 pagesSkin Care Creams, Lotions and Gels For Cosmetic Use - SpecificationJona Phie Montero NdtcnursingNo ratings yet
- RISO RZ User GuideDocument112 pagesRISO RZ User GuideJojo AritallaNo ratings yet
- Chapter-4 Conditional and Iterative Statements in PythonDocument30 pagesChapter-4 Conditional and Iterative Statements in Pythonashishiet100% (1)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Water-Based Paint Formulations, Vol. 3From EverandWater-Based Paint Formulations, Vol. 3Rating: 4.5 out of 5 stars4.5/5 (6)
- Transformer: The Deep Chemistry of Life and DeathFrom EverandTransformer: The Deep Chemistry of Life and DeathRating: 4.5 out of 5 stars4.5/5 (13)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)