Professional Documents
Culture Documents
04 Inorganic Chemistry 18-03-2021
Uploaded by
pratik0 ratings0% found this document useful (0 votes)
21 views1 pageOriginal Title
04_Inorganic_Chemistry_18-03-2021
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
21 views1 page04 Inorganic Chemistry 18-03-2021
Uploaded by
pratikCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
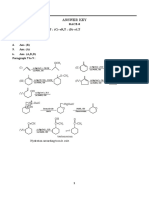
NEET - 2020-21
Chemistry
At a Eagle View
RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *RCC * *
Day-04 18-03-2020
Inorganic Chemistry viii) BaF2, SrF2, MgF2, CaF2, BeF2
ix) CaF2, CaCl2, CaBr2, CaI2
Important Increasing order
x) AgI, AgBr, AgCl, AgF 1
1. Acidic property Solubility
xi) PbO2, Cdl2, RbI covalent char.
i) SiO2, CO2, N2O5, SO3
6. Ionic Character
ii) MgO, Al2O3, SiO2, P4O10
i) LiBr, NaBr, KBr, RbBr, CsBr
iii) HClO, HClO2, HClO3, HClO4
ii) LiF, NaF, KF, RbF, CsF
iv) CH4, NH3, H2O, HF
iii) BeCl2, MgCl2, Ca SrCl2, BaCl2,
v) SiH4, PH3, H2S, HCl
iv) BCl3, AlCl3, GaCl3
vi) H2O, H2S, H2Se, H2Te
v) VCl4, VCl3, VCl2
vii)HF, HCl, HBr, HI
vi) AlF3, MgF2, NaF
viii) InCl3, GaCl3, AlCl3
vii)AlN, Al2O3, AlF3
ix) BF3, BCl3, BBr3 BI3 viii) HI, HBr, HCl, HF
2. Bond Angle ix) CuCN, AgCN
i) CH4, C2H4, C2H 2 ii) H2O, NH3, CH4, CO2 x) AgCl, KCl
iii) H2O, NH3, CH4, BH3 iv) NO2–, NO2, NO2+ 7. Atomic/Ionic Size
v) H2Se, H2S, H2O vi) AsH3, PH3, NH3 i) Mg2+, Na+, F–, O2–, M3– (Hint : Isoelectronic series)
vii) PF3, PCl3, PBr3, PI3 viii) NF3, NCl3 ii) Ca2+, Ar, Cl–, S2–
ix) NF3, NH3, NCl3 x) OF2, OH2, Cl2O iii) O, C, S, Se
3. Basic Character iv) B, Be, Li, Na
i) LiOH, NaOH, KOH, RbOH, CsOH v) F, O, F–, O2–
ii) Be(OH)2, Mg(OH)2, Ca(OH)2, Ba(OH)2 8. Oxidizing Power
iii) BeO, MgO, CaO, SrO i) Cr 2O72–, MnO4– ii) MnO42–, MnO4
iv) NiO, MgO, SrO, K2O, Cs2O iii) WO3, MoO3, CrO3 iv) GeCl4, SnCl4, PbCl4
v) CO2, B2O3, BeO, Li2O v) I2, Br2, Cl2, F2 vi) Zn+2, Fe+2, Pb2+, Cu2+, Ag+
vi) SiO2, Al2O3, MgO, Na2O 9. Ionization Energy
vii)SbH3, AsH3, PH3, NH3 i) Na, Al, Mg, Si
– – – –
viii) F , OH , NH2 , CH3 ii) Li, B, Be, C, O, N, F, Ne, He (Ist I.P.)
4. Thermal Stability iii) Be, C, B, N, F, O, Ne, Li, He (IInd I.P.)
i) Li2CO3, Na 2CO3, K 2CO3, Rb2CO3, Cs 2CO3 10. Melting Point
ii) BeCO3, MgCO3, CaCO3, BaCO3 i) Cs, Rb, K, Na, Li
iii) Be(OH)2, Mg(OH)2, Ca(OH)2, Sr(OH)2, Ba(OH)2 ii) Mg, Ba, Sr, Ca, Be
Polarisation iii) CaI2, CaBr2, CaCl2, CaF2
iv) LiOH, NaOH, KOH, RbOH, CsOH iv) BeCl2, MgCl2, CaCl2, SrCl2, BaCl2
v) BeSO4, MgSO4, CaSO4 v) NaI, NaBr, NaCl, NaF
vi) CsH, RbH, KH, NaH, LiH vi) CsCl, RbCl, KCl, NaCl
vii)SbH3, AsH3, PH3, NH3 vii)AlCl3, MgCl2, NaCl
viii) H2Te, H2Se, H2S, H2O 11. Density
ix) HI, HBr, HCl, HF i) Na, Al, Fe, Pb, Au ii) Li, K, Na, Rb, Cs
5. Solubility iii) Ca, Mg, Be, Sr, Ba iv) Highest Density = Os/Ir
i) BeCO3, CaCO3, MgCO3, BeCO3 v) Lowest density = H vi) Metal of lowest Density = Li
ii) Be(OH)2, Mg(OH)2, Ca(OH)2, Ba(OH)2 12. Boiling Point
iii) BaSO4, SrSO4, CaSO4, MgSO4, BeSO4 i) PH3, AsH3, NH3, SbH3 ii) H2S, H2Se, H2O
iv) Li2CO3, Na 2CO3, K2CO3, Rb2CO3, CsCO3 iii) HCl, HBr, HI, HF iv) NH3, HF, H2O
v) LiOH, NaOH, KOH, RbOH, CsOH v) He, Ne, Ar, Kr vi) H2O, D2O
vi) LiF, LiCl, LiBr, Lil vii)H2, Cl2, Br2
vii)LiF, NaF, KF, RbF, CsF
1
You might also like
- Sự Điện Li TopperDocument9 pagesSự Điện Li TopperKhanh NguyenNo ratings yet
- Chemical Bonding Part-2Document6 pagesChemical Bonding Part-2Nonu RajputNo ratings yet
- 03 Neutralization Reactions Worksheet KeyDocument2 pages03 Neutralization Reactions Worksheet KeySokahaNo ratings yet
- Neutralization Reactions Worksheet: Hi + Naoh H O + NaiDocument2 pagesNeutralization Reactions Worksheet: Hi + Naoh H O + NaiahaanNo ratings yet
- 10-Science FlowchartsDocument18 pages10-Science FlowchartsRaja Veeraiyan33% (3)
- LANGUAGE OF CHEM Worksheet 2023-24Document6 pagesLANGUAGE OF CHEM Worksheet 2023-24Chintan ShahNo ratings yet
- Foundations of College Chemistry 14th Edition Hein Solutions Manual DownloadDocument9 pagesFoundations of College Chemistry 14th Edition Hein Solutions Manual DownloadJohn Gaudreau100% (25)
- Nomenclature Worksheet NDocument2 pagesNomenclature Worksheet NVictor GarciaNo ratings yet
- Acid Base QuizDocument4 pagesAcid Base QuizHendrix Antonni AmanteNo ratings yet
- Qttsop: 1Os12Thzsoytlhdoztbasoy - Yphyyyyip.ODocument10 pagesQttsop: 1Os12Thzsoytlhdoztbasoy - Yphyyyyip.O33-Siddharth NairNo ratings yet
- Class04 Chemistry G11 Homework Sep 25-29Document4 pagesClass04 Chemistry G11 Homework Sep 25-29Erin100% (1)
- Skema Halus GaramDocument23 pagesSkema Halus GaramFAUZIAH BINTI HUYOP Moe100% (1)
- 1st Sem - 1st AssessDocument7 pages1st Sem - 1st Assessramya sekarNo ratings yet
- Chemical Formula & Names (Kamilia's Work)Document3 pagesChemical Formula & Names (Kamilia's Work)aina zahraaNo ratings yet
- Selina Concise Chemistry Class 9 ICSE Solutions For Chapter 1 - Language of ChemistryDocument24 pagesSelina Concise Chemistry Class 9 ICSE Solutions For Chapter 1 - Language of ChemistryfelixNo ratings yet
- Animes (Final) SheetDocument26 pagesAnimes (Final) SheetAnant JainNo ratings yet
- Kation Golongan IIIDocument1 pageKation Golongan IIIRaidhaNo ratings yet
- Chem (LAS)Document2 pagesChem (LAS)mhyrela roncedNo ratings yet
- Calventas Lab ReportDocument5 pagesCalventas Lab ReportGodwayneNo ratings yet
- FormulaDocument6 pagesFormulaLars RembrandtNo ratings yet
- Gr. 11U Review - D2L VersionDocument2 pagesGr. 11U Review - D2L Versionsar2005No ratings yet
- Phchem 1B - Quiz #1 - Chemical Nomenclature (Summer 2022)Document2 pagesPhchem 1B - Quiz #1 - Chemical Nomenclature (Summer 2022)Shopifyy ClothingNo ratings yet
- Unit 4 Naming Amp Types Naming Polyatomic Compounds Worksheet 2 Page 1 AnswersDocument2 pagesUnit 4 Naming Amp Types Naming Polyatomic Compounds Worksheet 2 Page 1 AnswersdiahemaNo ratings yet
- ACTIVITY NO. 2 (CHEMISTRY LABORATORY) - Chemistry For EngineersDocument2 pagesACTIVITY NO. 2 (CHEMISTRY LABORATORY) - Chemistry For EngineersArvhenn BarcelonaNo ratings yet
- 03 Neutralization Reactions WorksheetDocument2 pages03 Neutralization Reactions Worksheetapi-298247873No ratings yet
- 03 Neutralization Reactions WorksheetDocument2 pages03 Neutralization Reactions Worksheetapi-298247873No ratings yet
- Problem Set 3 NomenclatureDocument3 pagesProblem Set 3 NomenclatureKê VîňNo ratings yet
- CY5151 As1Document1 pageCY5151 As1Chris JonathanNo ratings yet
- S Bllock - 4 RevisedDocument3 pagesS Bllock - 4 RevisedAbhiNo ratings yet
- Neutralization Reactions Worksheet: A) Csoh + H Co + B) HF + MG (Oh) + C) Hno + Al (Oh) + D) HCL + Koh + E) Hbro + LiohDocument2 pagesNeutralization Reactions Worksheet: A) Csoh + H Co + B) HF + MG (Oh) + C) Hno + Al (Oh) + D) HCL + Koh + E) Hbro + LiohShammany AlsuwaidiiNo ratings yet
- BES111 LAB Act.2 Worksheet On Chemical Nomenclature 1Document2 pagesBES111 LAB Act.2 Worksheet On Chemical Nomenclature 1Ahmed Dhempsey Hali AbdulbasikNo ratings yet
- ACTIVITY NO. 2 (CHEMISTRY LABORATORY) - Chemistry For EngineersDocument2 pagesACTIVITY NO. 2 (CHEMISTRY LABORATORY) - Chemistry For EngineersArvhenn BarcelonaNo ratings yet
- Road Maps Organic Chemistry Set 2 Eklavya @JEEAdvanced - 2024 (2) (4 Files Merged)Document11 pagesRoad Maps Organic Chemistry Set 2 Eklavya @JEEAdvanced - 2024 (2) (4 Files Merged)puneethrgcNo ratings yet
- Race 8 Oc Answer KeyDocument2 pagesRace 8 Oc Answer KeyMat phixNo ratings yet
- Unit 7 Homework - Chemistry11Document10 pagesUnit 7 Homework - Chemistry11NameNo ratings yet
- Practice NomenclatureDocument1 pagePractice Nomenclaturerayan.ashroffNo ratings yet
- Hydrogen & S-Block Elements - DTS-0Document2 pagesHydrogen & S-Block Elements - DTS-0Rohan SrivastavaNo ratings yet
- 12th Chemistry (EM) 2022-2023 SampleDocument25 pages12th Chemistry (EM) 2022-2023 Sample11B CHARAN ANANDNo ratings yet
- Solubility Table Worksheet PDFDocument2 pagesSolubility Table Worksheet PDFCed Hernandez100% (1)
- Css ChemistryII 2017Document3 pagesCss ChemistryII 2017Bakhita MaryamNo ratings yet
- Chemistry Paper With AnswerDocument7 pagesChemistry Paper With AnswerGaurav MehtaNo ratings yet
- Memorization QuizDocument1 pageMemorization QuizBrenda SchroederNo ratings yet
- Naming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument5 pagesNaming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedManohar GarimellaNo ratings yet
- Balancing Chemical Equations With KeyDocument4 pagesBalancing Chemical Equations With Keymariam miladNo ratings yet
- Unit 2 ReviewDocument1 pageUnit 2 ReviewShun SatoNo ratings yet
- Alde & Ket-2&3Document14 pagesAlde & Ket-2&3ayesha sheikhNo ratings yet
- Naming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument5 pagesNaming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights Reservedpao manaligodNo ratings yet
- Naming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument5 pagesNaming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedMca ImusNo ratings yet
- Naming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument5 pagesNaming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedJaiy HingcoNo ratings yet
- Redox Reactionstest PDFDocument1 pageRedox Reactionstest PDFaleena'No ratings yet
- Control 3 - PCMDocument2 pagesControl 3 - PCMJuanito EmilioNo ratings yet
- Ionic Equ. (LDA) NMDocument18 pagesIonic Equ. (LDA) NMkaeshav manivannanNo ratings yet
- Neutralization Reactions WorksheetDocument2 pagesNeutralization Reactions Worksheetsamar alassadNo ratings yet
- Chapter 6 Part 2 (KSSM) - 231019 - 184442Document16 pagesChapter 6 Part 2 (KSSM) - 231019 - 184442Pewdan AsianNo ratings yet
- I. Multiple Choice Questions (Type-I)Document9 pagesI. Multiple Choice Questions (Type-I)Pratishtha KumariNo ratings yet
- I. Multiple Choice Questions (Type-I)Document9 pagesI. Multiple Choice Questions (Type-I)AamerNo ratings yet
- Alde & Ket-6&7Document15 pagesAlde & Ket-6&7ayesha sheikhNo ratings yet
- Hsslive-Xi-Chem-Ch-10. S-Block Elements-SignedDocument7 pagesHsslive-Xi-Chem-Ch-10. S-Block Elements-SignedMuhammed Sadiq100% (1)
- Charge of ElementsDocument1 pageCharge of ElementsKagarine__LarousseNo ratings yet
- Unit-Wise Weightage From NEET Exam 2020Document5 pagesUnit-Wise Weightage From NEET Exam 2020pratikNo ratings yet
- Chemistry: NEET - 2020-21Document1 pageChemistry: NEET - 2020-21pratikNo ratings yet
- Chemistry: NEET - 2020-21Document1 pageChemistry: NEET - 2020-21pratikNo ratings yet
- 05 Haloform Test 19-03-2021Document1 page05 Haloform Test 19-03-2021pratikNo ratings yet
- Jis Tieu Chuan NhatDocument2 pagesJis Tieu Chuan Nhatanhhoack3No ratings yet
- S Block Ncert SolutionsDocument32 pagesS Block Ncert SolutionsManish ShuklaNo ratings yet
- CH 14 PDFDocument26 pagesCH 14 PDFkrishnaNo ratings yet
- Combined Past Paper Questions On StoiciometryDocument27 pagesCombined Past Paper Questions On StoiciometryRamesh Iyer50% (4)
- 2017-GCWUF-3350 SUMAIRA SAIF (Roll# 189) Final Assignment (1) Inorganic ChemistDocument5 pages2017-GCWUF-3350 SUMAIRA SAIF (Roll# 189) Final Assignment (1) Inorganic ChemistSumaira SaifNo ratings yet
- Laeticia Rodrigues - P21008 - MetullurgyDocument2 pagesLaeticia Rodrigues - P21008 - MetullurgyLAETICIA RODRIGUESNo ratings yet
- Chapter - 4: Structure of The AtomDocument15 pagesChapter - 4: Structure of The Atomuma mishraNo ratings yet
- © 2013 Marshall Cavendish International (Singapore) Private LimitedDocument12 pages© 2013 Marshall Cavendish International (Singapore) Private LimitedKaung Myat SanNo ratings yet
- Solving Problem Involving Calculation by Chemical Equation2Document4 pagesSolving Problem Involving Calculation by Chemical Equation2aidarahim0205No ratings yet
- A Level Chemistry Paper 1 Set 1Document20 pagesA Level Chemistry Paper 1 Set 1RUBANGAKENE DENISNo ratings yet
- Chemical MandalaDocument2 pagesChemical MandalaScribdTranslationsNo ratings yet
- How Many Different Uses of Metal Can You Spot?Document47 pagesHow Many Different Uses of Metal Can You Spot?safiraginaNo ratings yet
- 9701 w06 QP 1Document16 pages9701 w06 QP 1Abdul QuddosNo ratings yet
- Short Tricks To Learn Periodic Table Easy To Learn Elements SSC CGL 2016 PDFDocument4 pagesShort Tricks To Learn Periodic Table Easy To Learn Elements SSC CGL 2016 PDFELANGOVAN VNo ratings yet
- Balancing Equations 31 PDFDocument5 pagesBalancing Equations 31 PDFIgnacio Jr. PaguyoNo ratings yet
- Sulphur SS2 2024 - 092511Document12 pagesSulphur SS2 2024 - 092511lindaoeghagharaNo ratings yet
- Common Metallurgical Defects in Grey Cast IronDocument9 pagesCommon Metallurgical Defects in Grey Cast IronRolando Nuñez Monrroy100% (1)
- Energy Chnages in Chemical ReactionsDocument40 pagesEnergy Chnages in Chemical Reactionsmuhammadshadid4No ratings yet
- THE PEROIDIC TABLE Answer Key 2dd55 61635c8cDocument1 pageTHE PEROIDIC TABLE Answer Key 2dd55 61635c8cbhagat johnsonNo ratings yet
- Mytest Coba ChemistryDocument3 pagesMytest Coba ChemistryDigilib Cambridge TazkiaNo ratings yet
- Humayra Hannan - Beginning BalancingDocument5 pagesHumayra Hannan - Beginning BalancingDivya SinghNo ratings yet
- Task 1 Reacting Masses 3Document2 pagesTask 1 Reacting Masses 3harishthestudent2No ratings yet
- Freight Ganesh ShivareDocument1 pageFreight Ganesh Shivarejadhavamit007No ratings yet
- Kimia T4 2023 - DLPDocument10 pagesKimia T4 2023 - DLPbrendan chee junNo ratings yet
- Answers For REINFORCEMENT EXERCISEDocument5 pagesAnswers For REINFORCEMENT EXERCISEAbgyyg LuRf UNo ratings yet
- MetalsDocument7 pagesMetalschongkee56No ratings yet
- Industrial Nitrogen Compounds & ExplosivesDocument118 pagesIndustrial Nitrogen Compounds & ExplosivesValentijn BrandNo ratings yet
- Mole-Mole WorksheetDocument2 pagesMole-Mole Worksheetzarna nirmal rawalNo ratings yet
- Supplement Catalogue 2013 en PDFDocument832 pagesSupplement Catalogue 2013 en PDFArvind KushwahaNo ratings yet
- Specific Heat of MetalsDocument3 pagesSpecific Heat of MetalsSukhjeet SinghNo ratings yet