Professional Documents
Culture Documents
Chemical Formula & Names (Kamilia's Work)
Uploaded by
aina zahraaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemical Formula & Names (Kamilia's Work)
Uploaded by
aina zahraaCopyright:
Available Formats
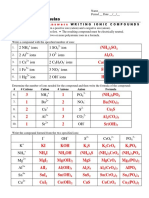
CHEMICAL FORMULA OF IONIC COMPOUNDS & THEIR NAMES
TABLE 1: WRITING THE CHEMICAL FORMULA OF THE IONIC COMPOUNDS
Sulphate Hydroxide Phosphate Carbonate Manganate(VII) Thiosulphate Dichromate
*ECS Fluoride ion Nitrate ion Chloride ion Bromide ion Iodide ion Oxide ion
ion ion ion ion ion ion (VI) ion
+\- F- SO42- NO3- Cl- Br- I- OH- PO43- CO32- O2- MnO4- S2O32- Cr2O72-
K+ KF K2SO4 KNO3 KCI KBr KI KOH K3PO4 K2CO3 K2O K2MnO4 K2S2O3 K2Cr2O7
Na NaF Na2SO4 NaNO3 NaCI NaBr NAI NaOH Na3PO4 Na2CO3 Na2O Na2MnO4 Na2S2O3 Na2Cr2O
7
2+
Ca CaF2 CaSO4 Ca(NO3)2 CaCI2 CaBr2 CAI2 CaOH2 Ca3(PO4)2 Ca2(CO3)2 CaO Ca(MnO4)2 CaS2O3 CaCr2O7
MgCr2O
Mg2+ MgF2 MgSO4 Mg(NO3)2 MgCI2 MgBr2 MgI2 MgOH2 Mg3(PO4)2 Mg2(CO3)2 MgO Mg(MnO4)2 MgS2O3
7
Al3+ AIF3 AI2(SO4)3 AI(NO3)3 AICI3 AIBr3 AII3 AIOH3 AI3(PO4)3 AI2(CO3)3 AI2O3 AI(MnO4)3 AI2(S2O3)3 AI2(Cr2O7)3
Zn2+ ZnF2 ZnSO4 Zn(NO3)2 ZnCI2 ZnBr2 ZnI2 ZnOH2 Zn3(PO4)2 Zn2(CO3)2 ZnO Zn(MnO4)2 ZnS2O3 ZnCr2O7
Fe2+ FeF2 FeSO4 Fe(NO3)2 FeCI2 FeBr2 FeI2 FeOH2 Fe3(PO4)2 Fe2(CO3)2 FeO Fe(MnO4)2 FeS2O3 FeCr2O7
Sn2+ SnF2 SnSO4 Sn(NO3)2 SnCI2 SnBr2 SnI2 SnOH2 Sn3(PO4)2 Sn2(CO3)2 SnO SN(MnO4)2 SnS2O3 SnCr2O7
Pb2+ PbF2 PbSO4 Pb(NO3)2 PbCI2 PbBr2 PbI2 PbOH2 Pb3(PO4)2 Pb2(CO3)2 PbO Pb(MnO4)2 PbS2O3 PbCr2O7
H+ HF H2SO4 HNO3 HCI HBr HI HOH H3PO4 H2CO3 H2O HMnO4 H2S2O3 H2Cr2O7
Cu2+ CuF2 CuSO4 Cu(NO3)2 CuCI2 CuBr2 CuI2 CuOH2 Cu3(PO4)2 Cu2(CO3)2 CuO Cu(MnO4)2 CuS2O3 CuCr2O7
Ag2Cr2O
Ag+ AgF Ag2SO4 AgNO3 AgCI AgBr AgI AgOH Ag3PO4 Ag2CO3 Ag2O AgMnO4 Ag2S2O3
7
NH4+
*E Manganate Thiosulphate Dichromate(VI)
Fluoride ion Sulphate ion Nitrate ion Chloride ion Bromide ion Iodide ion Hydroxide ion Phosphate ion Carbonate ion Oxide ion
CS (VII) ion ion ion
+
\ F- SO42- NO3- Cl- Br- I- OH- PO CO32- O2- MnO4- S2O32- Cr2O72-
-
Potassium Potassium Potassium Potassium Potassiu Potassium Potassium Potassium Potassiu Potassiu Potassiu
Potassiu Potassium
+ sulphate nitrate chloride bromide m iodide hydroxide phosphate carbonate m oxide m m
K m dichromate
mangan thiosulph
fluoride (VI)
ate ate
Sodium Sodium Sodium
N Sodium Sodium Sodium sodium Sodium Sodium Sodium Sodium Sodium Sodium
mangan thiosulph dichromate
a+ fluoride sulphate nitrate chloride bromide iodide hydroxide phosphate carbonate oxide
ate ate (VI)
C Calcium Calcium Calcium
Calcium Calcium Calcium calcium Calcium Calcium Calcium Calcium Calcium Calcium
a2 mangan thiosulph dichromate
+ fluoride sulphate nitrate chloride bromide iodide hydroxide phosphate carbonate oxide
ate ate (VI)
Magnesium Magnesium magnesium Magnesium Magnesiu Magnesium Magnesium Magnesium Magnesiu Magnesi Magnesiu
M Magnesiu Magnesium
sulphate nitrate chloride bromide m iodide hydroxide phosphate carbonate m oxide um m
g2 m dichromate
+ mangan thiosulph
fluorine (VI)
ate ate
Al Aluminium aluminium aluminium Aluminium Aluminiu Aluminium Aluminium Aluminiu Alumini Aluminiu
3+ Aluminiu Aluminium
sulphate nitrate chloride bromide m iodide hydroxide phosphate m oxide um m
m Aluminium dichromate
mangan thiosulph
fluorine (VI)
ate ate
Z Zinc Zinc Zinc
Zinc Zinc Zinc Zinc Zinc Zinc Zinc Zinc Zinc Zinc
n2 mangan thiosulph dichromate
+ flourine sulphate nitrate chloride bromide iodide hydroxide phosphate carbonate oxide
ate ate (VI)
F Iron Iron Iron
Iron Iron Iron Iron Iron Iron Iron Iron Iron
e2 Iron oxide mangan thiosulph dichromate
+ fluorine sulphate nitrate chloride bromide iodide hydroxide phosphate carbonate
ate ate (VI)
S Tin Tin Tin
Tin Tin Tin Tin Tin Tin
n2 Tin nitrate Tin bromide Tin iodide Tin oxide mangan thiosulph dichromate
+ fluorine sulphate chloride hydroxide phosphate carbonate
ate ate (VI)
P Lead Lead Lead
Lead Lead Lead Lead Lead Lead Lead Lead Lead
b2 lead nitrate mangan thiosulph dichromate
+ fluorine sulphate chloride bromide iodide hydroxide phosphate carbonate oxide
ate ate (VI)
Hydroge
Hydrogen Hydrogen
Hydrogen Hydrogen hydrogen Hydrogen Hydrogen Hydrogen Hydrogen Hydrogen Hydrogen Hydrogen n
H+ thiosulph dichromate
fluorine sulphate nitrate chloride bromide iodide hydroxide phosphate carbonate oxide mangan
ate (VI)
ate
C Copper copper copper
Copper Copper copper Copper Copper Copper Copper copper Copper copper
u2 mangan thiosulph dichromate
+ fluorine sulphate nitrate chloride bromide iodide hydroxide phosphate carbonate oxide
ate ate (VI)
Silver Silver Silver
A Silver Silver silver Silver Silver Silver Silver Silver Silver Silver
mangan thiosulph dichromate
g+ fluorine sulphate nitrate chloride bromide iodide hydroxide phosphate carbonate oxide
ate ate (VI)
N
H4
+
TABLE 2: WRITING THE NAMES OF THE IONIC COMPOUNDS
You might also like
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsFrom EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsNo ratings yet
- Chemical Formula Writing Worksheet PDFDocument4 pagesChemical Formula Writing Worksheet PDFkezia0% (1)
- The Modern Spanish: Breyer and Zaitsev SystemsFrom EverandThe Modern Spanish: Breyer and Zaitsev SystemsRating: 5 out of 5 stars5/5 (1)
- COLOUROf IONICCOMPOUNDSDocument2 pagesCOLOUROf IONICCOMPOUNDSkrutika goharkarNo ratings yet
- Materials Data for Cyclic Loading: Low-Alloy SteelsFrom EverandMaterials Data for Cyclic Loading: Low-Alloy SteelsRating: 5 out of 5 stars5/5 (2)
- Naming & Balancing Chemical Formula - Sheet1Document1 pageNaming & Balancing Chemical Formula - Sheet1arseniy kraschenkoNo ratings yet
- IonicBonding WritingFormulas WKST KEYDocument2 pagesIonicBonding WritingFormulas WKST KEYMaria Isabel DicoNo ratings yet
- Cajepe, Cherry May F. Bses 1a ChemistryDocument4 pagesCajepe, Cherry May F. Bses 1a ChemistryNilda FranciscoNo ratings yet
- Problem Set No. 1Document3 pagesProblem Set No. 1Ej Ferrer100% (1)
- JRS Tutorials: Chemistry IITDocument58 pagesJRS Tutorials: Chemistry IITtusharr11.mobNo ratings yet
- Unit 4 Naming Amp Types Naming Polyatomic Compounds Worksheet 2 Page 1 AnswersDocument2 pagesUnit 4 Naming Amp Types Naming Polyatomic Compounds Worksheet 2 Page 1 AnswersdiahemaNo ratings yet
- 7 - Chemical Formulas: AnswersDocument1 page7 - Chemical Formulas: AnswersAlyssa Mae MayonadoNo ratings yet
- Heating Effect of Carbonate & Bicarbonate SaltsDocument3 pagesHeating Effect of Carbonate & Bicarbonate Saltsvishwajit patilNo ratings yet
- Formula Writing - CambridgeDocument5 pagesFormula Writing - CambridgeQusai Saify100% (3)
- Heating Effects (12th&13th)Document4 pagesHeating Effects (12th&13th)Raju SinghNo ratings yet
- Heating Effect ReactionsDocument3 pagesHeating Effect ReactionsGautam SharmaNo ratings yet
- Phchem 1B - Quiz #1 - Chemical Nomenclature (Summer 2022)Document2 pagesPhchem 1B - Quiz #1 - Chemical Nomenclature (Summer 2022)Shopifyy ClothingNo ratings yet
- Lesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li ODocument16 pagesLesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li OThai NgoNo ratings yet
- Calventas Lab ReportDocument5 pagesCalventas Lab ReportGodwayneNo ratings yet
- ,, ,, 2 2, ,, ,, ,, ,,, , ,,, 2 2, ,, ,, ,, ,,, ,, ,,, ,, ,, ,, ,, ,, ,, ,,, 8N HnoDocument6 pages,, ,, 2 2, ,, ,, ,, ,,, , ,,, 2 2, ,, ,, ,, ,,, ,, ,,, ,, ,, ,, ,, ,, ,, ,,, 8N HnoPrajwal TalwalkarNo ratings yet
- Write The Formulas For The Following Ionic Compounds:: Bonding and Naming WS 4Document2 pagesWrite The Formulas For The Following Ionic Compounds:: Bonding and Naming WS 4Bea Lha Zandra BesingaNo ratings yet
- Core UNIT 4 - Assignment 2 - Writing Formulas For Ionic CompoundsDocument3 pagesCore UNIT 4 - Assignment 2 - Writing Formulas For Ionic CompoundsCrystal R.MNo ratings yet
- Ions - MemorizeDocument1 pageIons - MemorizeThea Clarice AmlonNo ratings yet
- CW 4 Unit 5 - Chemical FormulaDocument1 pageCW 4 Unit 5 - Chemical Formulamohammad hasanNo ratings yet
- No Kation Nama No Anion NamaDocument2 pagesNo Kation Nama No Anion NamaAjeng Candra Arum PuspasariNo ratings yet
- Review Problems Chapter 4 Solutions PDFDocument4 pagesReview Problems Chapter 4 Solutions PDFAntoninoNo ratings yet
- Syed ArsalanDocument1 pageSyed ArsalanShahzaib MughalNo ratings yet
- For JEE Aspirants: Complete Inorganic Chemistry ReactionsDocument56 pagesFor JEE Aspirants: Complete Inorganic Chemistry ReactionsLakshmi AnandNo ratings yet
- Worksheet RedoxDocument4 pagesWorksheet RedoxSyed asif HaleemNo ratings yet
- (VCE Chemistry) 2017 BC Unit 2 Data SheetDocument4 pages(VCE Chemistry) 2017 BC Unit 2 Data SheetJustine LyNo ratings yet
- GenChem Nomenclature Updated PDFDocument2 pagesGenChem Nomenclature Updated PDFCamille AquinoNo ratings yet
- Chapter 8: SaltsDocument23 pagesChapter 8: SaltsWong Wai LunNo ratings yet
- Formulas of Compounds Polyatomics KEYDocument2 pagesFormulas of Compounds Polyatomics KEYJewel Emerald C. CudiamatNo ratings yet
- IOC All ReactionsDocument56 pagesIOC All ReactionsKeerthana MNo ratings yet
- Redox Reaction BalancingDocument9 pagesRedox Reaction BalancingAman9692No ratings yet
- CH 6 Name For The Formula ShownDocument1 pageCH 6 Name For The Formula Showntownsenr94No ratings yet
- Compound SudokuDocument1 pageCompound Sudokudaisuke ʕ•ᴥ•ʔNo ratings yet
- Formula Kimia Dan Nama Bahan KimiaDocument2 pagesFormula Kimia Dan Nama Bahan Kimiahansm100% (5)
- Exp1 Prelab Day1Document2 pagesExp1 Prelab Day1Aaron OrtegaNo ratings yet
- Inorganic Nomenclature Rules, Examples and Practice Problems Bansal PatternDocument7 pagesInorganic Nomenclature Rules, Examples and Practice Problems Bansal PatternKumarNo ratings yet
- Ion Memorization ListDocument2 pagesIon Memorization Listdchao94No ratings yet
- ChemistryDocument1 pageChemistrydanamvasquezNo ratings yet
- Class04 Chemistry G11 Homework Sep 25-29Document4 pagesClass04 Chemistry G11 Homework Sep 25-29Erin100% (1)
- Types of Chemical ReactionsDocument2 pagesTypes of Chemical ReactionsHimeNo ratings yet
- Elements (Anions) Symbol Oxidation NO. Elements (Anions) Symbol Oxidation NODocument5 pagesElements (Anions) Symbol Oxidation NO. Elements (Anions) Symbol Oxidation NOJims Cudinyerah100% (1)
- Oxidation and Reduction-1 (13Document1 pageOxidation and Reduction-1 (13Aditya ChudasamaNo ratings yet
- တက္ကသိုလ်ဝင်တန်း ဓာတုဗေဒ Dr.Soe Kyaw KyawDocument322 pagesတက္ကသိုလ်ဝင်တန်း ဓာတုဗေဒ Dr.Soe Kyaw KyawKhin OosweNo ratings yet
- 2022 2023 General Chemistry I Study Question Set 2Document1 page2022 2023 General Chemistry I Study Question Set 2Ömer Burak YükselNo ratings yet
- Kimia Unsur OkDocument38 pagesKimia Unsur OkUntuk ViuNo ratings yet
- Symbol and Charges For Monoatomic and Polyatomic Ions, Oxidation Number, and Acid NamesDocument3 pagesSymbol and Charges For Monoatomic and Polyatomic Ions, Oxidation Number, and Acid NamesKelvin Mark KaabayNo ratings yet
- Chapter 20 Worksheet Redox WSDocument4 pagesChapter 20 Worksheet Redox WSMostafa Ahmed100% (1)
- Chemical Bonding: Why Bond Anyway?Document45 pagesChemical Bonding: Why Bond Anyway?PutRi Charolin GintingNo ratings yet
- AP Chemistry Summer Assignment 2017Document44 pagesAP Chemistry Summer Assignment 2017John SmithNo ratings yet
- Common Polyatomic IonsDocument1 pageCommon Polyatomic IonsRoddyNo ratings yet
- Formula Writing and Naming of CompoundsDocument1 pageFormula Writing and Naming of CompoundsMon ColinaNo ratings yet
- COLOUR OF ALL IOC COMPOUNDS @HeyitsyashXDDocument2 pagesCOLOUR OF ALL IOC COMPOUNDS @HeyitsyashXDzehraNo ratings yet
- Skema Halus GaramDocument23 pagesSkema Halus GaramFAUZIAH BINTI HUYOP Moe100% (1)
- MANTARA - Docx ACTIVITY#5 PART BDocument3 pagesMANTARA - Docx ACTIVITY#5 PART BFarks Mantara0% (1)
- Chemical ReactionsDocument6 pagesChemical ReactionsKushNo ratings yet
- Cations & Anions WorksheetDocument1 pageCations & Anions WorksheetGapor examNo ratings yet
- PH110-CHAPTER 5 Linear Momentum and CollisionsDocument8 pagesPH110-CHAPTER 5 Linear Momentum and CollisionsNtape Knox SiwaleNo ratings yet
- ACFrOgDsH3VyGwJDuLIBt9Sb 9enHJVEr0wP6B4wcrzTyWYmDY0qum5oJr 24rwY8FiIS46xaikTY 0o2CHJ8zrKcxUSEUp5hET5mc If6XGNyx28ua5LgMCy1VggwoEOSEFMwCl2mncwNfWGIq7Document10 pagesACFrOgDsH3VyGwJDuLIBt9Sb 9enHJVEr0wP6B4wcrzTyWYmDY0qum5oJr 24rwY8FiIS46xaikTY 0o2CHJ8zrKcxUSEUp5hET5mc If6XGNyx28ua5LgMCy1VggwoEOSEFMwCl2mncwNfWGIq7Rudiery ReisNo ratings yet
- Argco Fig 403 - Mec TeeDocument3 pagesArgco Fig 403 - Mec TeeFIRE RL SYSTEMSNo ratings yet
- BibliographyDocument2 pagesBibliographyAdhi HutariNo ratings yet
- Chemistry Project: Organic PreparationDocument12 pagesChemistry Project: Organic PreparationAthul Oscar RonaldoNo ratings yet
- RLC TransientDocument12 pagesRLC TransientFlorenzo Miguel AclanNo ratings yet
- Literature Review of Solar Power PlantDocument8 pagesLiterature Review of Solar Power Plantbav1dik0jal3100% (1)
- Introduction To Flow Cytometry May 10 PDFDocument7 pagesIntroduction To Flow Cytometry May 10 PDFSilviaGonzalezSierraNo ratings yet
- Green ChemistryDocument12 pagesGreen ChemistrymidhunNo ratings yet
- HCS02 HCS03 Project Planning ManualDocument312 pagesHCS02 HCS03 Project Planning ManualleandroNo ratings yet
- Dokumen - Tips - Pompe de Caldura BuclaDocument96 pagesDokumen - Tips - Pompe de Caldura BuclaAnonymous oKTCFZNTmNo ratings yet
- Boiler Water Treatment: Thermax Limited Chemical DivisionDocument53 pagesBoiler Water Treatment: Thermax Limited Chemical Divisionkcp1986100% (2)
- Options For High Temperature Well StimulationDocument11 pagesOptions For High Temperature Well StimulationFra FraNo ratings yet
- CSM Technical SpecDocument1 pageCSM Technical SpecabasakNo ratings yet
- Technical Data Sheet: Additional Solder Alloys Manufactured by Nathan Trotter Chemical Specifications Physical PropertiesDocument2 pagesTechnical Data Sheet: Additional Solder Alloys Manufactured by Nathan Trotter Chemical Specifications Physical PropertiesGossai HanafiNo ratings yet
- Q6f559Brev1 DRGDocument1 pageQ6f559Brev1 DRGVivek SavaliyaNo ratings yet
- 6mm Hole DeagssingDocument10 pages6mm Hole DeagssingAkintoye AsaoluNo ratings yet
- Hazardous Waste ManagementDocument20 pagesHazardous Waste ManagementYuki SalemNo ratings yet
- OlymphysicsDocument6 pagesOlymphysicslokiiiNo ratings yet
- Doc-20231126-Wa0008. 20231126 161647 0000Document13 pagesDoc-20231126-Wa0008. 20231126 161647 0000chanchal.x04No ratings yet
- Acronal PLUS 4641: Formulation 4641-010Document2 pagesAcronal PLUS 4641: Formulation 4641-010Thanh VuNo ratings yet
- Field Test of Construction MaterialsDocument25 pagesField Test of Construction MaterialsAntarjyami PradhanNo ratings yet
- Conservation of MomentumDocument23 pagesConservation of MomentumPauling ChiaNo ratings yet
- D 2251 - 96 R00 - RdiynteDocument2 pagesD 2251 - 96 R00 - RdiynteRuben YoungNo ratings yet
- Air Conditioner: Service ManualDocument81 pagesAir Conditioner: Service ManualLenka CapovaNo ratings yet
- Circular Motion PhysicsDocument4 pagesCircular Motion Physicsdhion13No ratings yet
- Buoyancy and StabilityDocument56 pagesBuoyancy and StabilityJuan Sebastian Varela SanabriaNo ratings yet
- MSDS Easy-Clean - 20Document3 pagesMSDS Easy-Clean - 20Ninh ChinhNo ratings yet
- QC Chart - Graphite AAS - DRAFTDocument13 pagesQC Chart - Graphite AAS - DRAFTConsultant JerocasNo ratings yet
- Literature ReviewDocument26 pagesLiterature ReviewMark Geoshua GodoyNo ratings yet