Professional Documents
Culture Documents
Exp1 Prelab Day1
Uploaded by
Aaron Ortega0 ratings0% found this document useful (0 votes)
186 views2 pagesOriginal Title
exp1_prelab_day1
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
186 views2 pagesExp1 Prelab Day1
Uploaded by
Aaron OrtegaCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
Ortega, Aaron
Chem 1B Section 7
Experiment 1 – Prelab
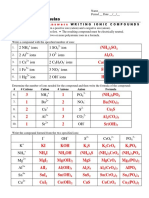
Table 1 - Prediction of Solubility of Salts in water
Cl- Br- I- SO4 2- PO4 3- OH- CO3 2- C2H3O 2- CrO4 2-
(S) (S) (S) (S) (S) (S) (S) (S) (S)
Na+ NaCl NaBr NaI Na2SO4 Na3PO4 NaOH Na2CO3 NaC2H3O2 Na2CrO4
(S) (S) (S) (S) (S) (S) (S) (S) (S)
K+ KCl KBr KI K2SO4 K3PO4 KOH K2CO3 KC2H3O2 K2CrO4
(S) (S) (S) (S) (S) (S) (S) (S) (S)
NH4+ NH4Cl NH4Br NH4I (NH4)2SO4 (NH4)3PO4 NH4OH (NH4)2CO3 NH4C2H3O2 (NH4)2CrO4
(S) (S) (S) (I) (I) (S) (I) (S) (I)
Ca2+ CaCl2 CaBr2 CaI2 CaSO4 Ca3(PO4)2 Ca(OH)2 CaCO3 Ca(C2H3O2)2 CaCrO4
(I) (S)
Mg2 (S) (S) (S) (S) Mg3(PO4) (I) (I) Mg(C2H3O2) (I)
+ MgCl2 MgBr2 MgI2 MgSO4 2 Mg(OH)2 MgCO3 2 MgCrO4
(S) (S) (S) (I) (I) (S) (I) (S) (I)
Ba2+ BaCl2 BaBr2 BaI2 BaSO4 Ba3(PO4)2 Ba(OH)2 BaCO3 Ba(C2H3O2)2 BaCrO4
(S) (S) (S) (S) (I) (I) (I) (S) (I)
Cr3+ CrCl3 CrBr3 CrI3 Cr2(SO4)3 CrPO4 Cr(OH)3 Cr2(CO3)3 Cr(C2H3O2)3 Cr2(CrO4)3
(S) (S) (S) (S) (I) (I) (I) (S) (I)
Fe2+ FeCl2 FeBr2 FeI2 FeSO4 Fe3(PO4)2 Fe(OH)2 FeCO3 Fe(C2H3O2)2 FeCrO4
(S) (S) (S) (S) (I) (I) (I) (S) (I)
Co2+ CoCl2 CoBr2 CoI2 CoSO4 Co3(PO4)2 Co(OH)2 CoCO3 Co(C2H3O2)2 CoCrO4
(S) (S) (S) (S) (I) (I) (I) (S) (I)
Ni2+ NiCl2 NiBr2 NiI2 NiSO4 Ni3(PO4)2 Ni(OH)2 NiCO3 Ni(C2H3O2)2 NiCrO4
(S) (S) (S) (S) (I) (I) (I) (S) (I)
Cu2+ CuCl2 CuBr2 CuI2 CuSO4 Cu3(PO4)2 Cu(OH)2 CuCO3 Cu(C2H3O2)2 CuCrO4
(I) (I) (S) (I) (I) (I) (I) (I)
Ag+ AgCl (I) AgBr AgI Ag2SO4 Ag3PO4 AgOH Ag2CO3 AgC2H3O2 Ag2CrO4
(I) (I) (I) (I) (I) (I) (I) (S) (I)
Pb2+ PbCl2 PbBr2 PbI2 PbSO4 Pb3(PO4)2 Pb(OH)2 PbCO3 Pb(C2H3O2)2 PbCrO4
Ortega, Aaron
Chem 1B Section 7
Experiment 1 – Prelab
Table 2 – Solubility of Compounds in Water
Solid Name of Strong, Weak, Primary Predicted Solubility Solubility Lab
Compound compound Nonelectrolyte species Solubility in 100mL in HNO3 Observations
in H2O in H2O H2O
1a BaCl Barium Strong BaSO4 Insoluble 37.5 g -----
chloride
1b BaSO4 ----- ----- ----- ----- -----
2a Ca(NO3)2 Calcium soluble 121.2 g -----
nitrate
2b Ca3(PO4)2 ----- ----- ----- ----- -----
3 (NH4)2SO4 Ammonium 76.7 g -----
sulfate
4a PbCO3 Lead insoluble -----
carbonate
4b PbCO3 ----- ----- ----- ----- -----
5a AgC2H3O2 Silver 1.02 g -----
acetate
5b AgC2H3O2 ----- ----- ----- ----- -----
6 Hg2Cl2 Mercury (I) Strong Hg2Cl2 Insoluble 0.2 mg -----
chloride (s)
7a Cu2O Copper (I) insoluble -----
oxide
7b Cu2O ----- ----- ----- ----- -----
8 C6H12O6 glucose Non electrolyte soluble 91 g -----
9 NaHSO4 Sodium -----
bisulfate
10 KI Potassium 140 g -----
iodine
11 Benzoic acid Benzoic acid nonelectrolyte soluble 0.34 g -----
C6H5COOH
You might also like
- Cajepe, Cherry May F. Bses 1a ChemistryDocument4 pagesCajepe, Cherry May F. Bses 1a ChemistryNilda FranciscoNo ratings yet
- Formulas of Compounds Polyatomics KEYDocument2 pagesFormulas of Compounds Polyatomics KEYJewel Emerald C. CudiamatNo ratings yet
- COLOUROf IONICCOMPOUNDSDocument2 pagesCOLOUROf IONICCOMPOUNDSkrutika goharkarNo ratings yet
- Module SaltDocument12 pagesModule SaltAzie Nurul Akhtar100% (1)
- Chemical Formula Writing Worksheet PDFDocument4 pagesChemical Formula Writing Worksheet PDFkezia0% (1)
- Chapter 8: SaltsDocument23 pagesChapter 8: SaltsWong Wai LunNo ratings yet
- 12 DChem Research SolubilityDocument6 pages12 DChem Research SolubilityRenzelle MelisseNo ratings yet
- JRS Tutorials: Chemistry IITDocument58 pagesJRS Tutorials: Chemistry IITtusharr11.mobNo ratings yet
- BARIUM CHLORIDE Ex. 11Document6 pagesBARIUM CHLORIDE Ex. 11wizard hamdsNo ratings yet
- Write The Formulas For The Following Ionic Compounds:: Bonding and Naming WS 4Document2 pagesWrite The Formulas For The Following Ionic Compounds:: Bonding and Naming WS 4Bea Lha Zandra BesingaNo ratings yet
- Chemical Formula & Names (Kamilia's Work)Document3 pagesChemical Formula & Names (Kamilia's Work)aina zahraaNo ratings yet
- Worksheet - Solubility Rules With AnswersDocument2 pagesWorksheet - Solubility Rules With AnswersEmmani HaginsNo ratings yet
- Qualitative Analysis of Anions: Pre-Lab AssignmentDocument18 pagesQualitative Analysis of Anions: Pre-Lab AssignmentNaths BarreraNo ratings yet
- Solubility Table Worksheet PDFDocument2 pagesSolubility Table Worksheet PDFCed Hernandez100% (1)
- Eleazar - Quiz#3Document2 pagesEleazar - Quiz#3ゆかりNo ratings yet
- Basic Inorganic Chemistry PHR 125: Prof. Dr. Mona BedairDocument33 pagesBasic Inorganic Chemistry PHR 125: Prof. Dr. Mona BedairAvvari AnnamaniNo ratings yet
- IonicBonding WritingFormulas WKST KEYDocument2 pagesIonicBonding WritingFormulas WKST KEYMaria Isabel DicoNo ratings yet
- TUpload 2Document1 pageTUpload 2Burikaw GamingNo ratings yet
- 7 - Chemical Formulas: AnswersDocument1 page7 - Chemical Formulas: AnswersAlyssa Mae MayonadoNo ratings yet
- Precipitation Reactions NotesDocument8 pagesPrecipitation Reactions NotessprijayaNo ratings yet
- Formula Writing - CambridgeDocument5 pagesFormula Writing - CambridgeQusai Saify100% (3)
- Experiment 4 Qualitative Analysis of CationsDocument8 pagesExperiment 4 Qualitative Analysis of CationsUzo Paul NwabuisiNo ratings yet
- Lab Write Up From HellDocument5 pagesLab Write Up From HellJeoffNo ratings yet
- CW 4 Unit 5 - Chemical FormulaDocument1 pageCW 4 Unit 5 - Chemical Formulamohammad hasanNo ratings yet
- Solubility Rules Practice WorksheetDocument2 pagesSolubility Rules Practice WorksheetSarah Yetti0% (1)
- Salt AnalysisDocument4 pagesSalt AnalysisMarietta ChristopherNo ratings yet
- Skema Halus Persamaan Kimia - PDFDocument9 pagesSkema Halus Persamaan Kimia - PDFIza MohdSabri33% (3)
- Heating Effects (12th&13th)Document4 pagesHeating Effects (12th&13th)Raju SinghNo ratings yet
- 8A Salts - AnswerDocument14 pages8A Salts - AnswerWong Wai LunNo ratings yet
- Experiment No. 5 The Solubility of Common Salts in WaterDocument8 pagesExperiment No. 5 The Solubility of Common Salts in WaterJoyce Bensig CastilNo ratings yet
- Class04 Chemistry G11 Homework Sep 25-29Document4 pagesClass04 Chemistry G11 Homework Sep 25-29Erin100% (1)
- Solution of Salt Analysis-13thDocument8 pagesSolution of Salt Analysis-13thRaju SinghNo ratings yet
- Unit 4 Naming Amp Types Naming Polyatomic Compounds Worksheet 2 Page 1 AnswersDocument2 pagesUnit 4 Naming Amp Types Naming Polyatomic Compounds Worksheet 2 Page 1 AnswersdiahemaNo ratings yet
- Wan Noor Afifah BT Wan YusoffDocument33 pagesWan Noor Afifah BT Wan YusoffThilagavathyNo ratings yet
- Skema Halus GaramDocument23 pagesSkema Halus GaramFAUZIAH BINTI HUYOP Moe100% (1)
- AP Chemistry Summer Assignment 2017Document44 pagesAP Chemistry Summer Assignment 2017John SmithNo ratings yet
- 6 Precipitation ReactionsDocument2 pages6 Precipitation ReactionsJacob DaughertyNo ratings yet
- Syed ArsalanDocument1 pageSyed ArsalanShahzaib MughalNo ratings yet
- Heating Effect ReactionsDocument3 pagesHeating Effect ReactionsGautam SharmaNo ratings yet
- Data Sheet Revision PDFDocument2 pagesData Sheet Revision PDFShifa RizwanNo ratings yet
- Balancing Equations WorksheetsDocument5 pagesBalancing Equations WorksheetsRovie AbuevaNo ratings yet
- 2022 2023 General Chemistry I Study Question Set 2Document1 page2022 2023 General Chemistry I Study Question Set 2Ömer Burak YükselNo ratings yet
- Solution Stoichiometry - Andnetionic.answers 3Document2 pagesSolution Stoichiometry - Andnetionic.answers 3Rahill SafiNo ratings yet
- Kimia Unsur OkDocument38 pagesKimia Unsur OkUntuk ViuNo ratings yet
- Calventas Lab ReportDocument5 pagesCalventas Lab ReportGodwayneNo ratings yet
- Net Ionic EquationDocument3 pagesNet Ionic Equationsara bdeirNo ratings yet
- WEDNESDAY 12:00 - 2:00 PM: Oceña, Margarito Jr. ODocument8 pagesWEDNESDAY 12:00 - 2:00 PM: Oceña, Margarito Jr. ONivla GenesisNo ratings yet
- Heating Effect of Carbonate & Bicarbonate SaltsDocument3 pagesHeating Effect of Carbonate & Bicarbonate Saltsvishwajit patilNo ratings yet
- Chemical Equations: Preparation For College Chemistry Columbia University Department of ChemistryDocument31 pagesChemical Equations: Preparation For College Chemistry Columbia University Department of ChemistryLakshmi SinghNo ratings yet
- Precipitation RxnsDocument5 pagesPrecipitation RxnsSavie:D100% (1)
- Ion Memorization ListDocument2 pagesIon Memorization Listdchao94No ratings yet
- (VCE Chemistry) 2017 BC Unit 2 Data SheetDocument4 pages(VCE Chemistry) 2017 BC Unit 2 Data SheetJustine LyNo ratings yet
- Language of Chemistry: CH5 STD:7Document14 pagesLanguage of Chemistry: CH5 STD:7Aatman GargNo ratings yet
- Lesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li ODocument16 pagesLesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li OThai NgoNo ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsFrom EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsNo ratings yet
- Unusual Structures and Physical Properties in Organometallic ChemistryFrom EverandUnusual Structures and Physical Properties in Organometallic ChemistryNo ratings yet
- Materials Data for Cyclic Loading: Low-Alloy SteelsFrom EverandMaterials Data for Cyclic Loading: Low-Alloy SteelsRating: 5 out of 5 stars5/5 (2)
- Propagation Methods in PlantsDocument36 pagesPropagation Methods in PlantsThea PadlanNo ratings yet
- Applied Veterinary Clinical Nutrition 2Nd Edition Andrea J Fascetti Full ChapterDocument51 pagesApplied Veterinary Clinical Nutrition 2Nd Edition Andrea J Fascetti Full Chapterginny.hudson476100% (5)
- Gudeta Uma PDFDocument106 pagesGudeta Uma PDFkelid IbrahimNo ratings yet
- Idiomatic ExpressionDocument27 pagesIdiomatic ExpressionJess Jess100% (1)
- Lesson Plan Restaurant EnglishDocument10 pagesLesson Plan Restaurant Englishemma12161216100% (2)
- AbyysDocument31 pagesAbyysGoody's workNo ratings yet
- Economics 7th Edition Hubbard Test BankDocument155 pagesEconomics 7th Edition Hubbard Test BankCynthiaJordanMDkqyfj100% (19)
- Advance Baking 11 (Kinds Dough)Document54 pagesAdvance Baking 11 (Kinds Dough)M Merllan Mier100% (1)
- Grade 5 Unit3Document23 pagesGrade 5 Unit3quynhNo ratings yet
- Essay 34Document1 pageEssay 34fatinNo ratings yet
- GM - Session 1-Introduction To FunctionsDocument38 pagesGM - Session 1-Introduction To FunctionsPinagpalit SamalapitNo ratings yet
- Food Research International 136 (2020) 109516Document8 pagesFood Research International 136 (2020) 109516athoillah_JrNo ratings yet
- Food DictionaryDocument3 pagesFood DictionarywingsoflifeNo ratings yet
- Modal Verbs of SpeculationDocument8 pagesModal Verbs of SpeculationCarolina CherezNo ratings yet
- Vitamin K2 PaperDocument55 pagesVitamin K2 PapernbpravinNo ratings yet
- 08 March Wati HotelDocument18 pages08 March Wati Hoteluniformbucket07No ratings yet
- English Cub RiddlesDocument14 pagesEnglish Cub RiddlesRavvjiit NandayNo ratings yet
- A Mini Book of SpellsDocument50 pagesA Mini Book of SpellsSoft Feng100% (1)
- Englishs MSCDocument55 pagesEnglishs MSCAgnichandra SubediNo ratings yet
- EwDocument75 pagesEwEm DraperNo ratings yet
- Lesson Plan Procedure TextDocument12 pagesLesson Plan Procedure TextSiti ShalihaNo ratings yet
- Writing PracticeDocument36 pagesWriting Practicemary antonette colladoNo ratings yet
- Birds of Sri LankaDocument9 pagesBirds of Sri LankaKelum KonaraNo ratings yet
- Shahzaib Ahmed Shaikh K16CE15 Prium Dodeja K16CE32 Shaban Mahar K16CE58Document12 pagesShahzaib Ahmed Shaikh K16CE15 Prium Dodeja K16CE32 Shaban Mahar K16CE58SalikNo ratings yet
- HENJEL - LE TVi - Q4 - Perform Harvesting Using Appropriate Materials, Tools, and Equipment - 5.3Document5 pagesHENJEL - LE TVi - Q4 - Perform Harvesting Using Appropriate Materials, Tools, and Equipment - 5.3HENJEL PERALESNo ratings yet
- Oap Model AnswerDocument4 pagesOap Model AnswerLasaltech Trainers Methodology100% (2)
- TOEFL Practice 197 +Document60 pagesTOEFL Practice 197 +bajickaNo ratings yet
- Honey For Nutrition and Health A ReviewDocument35 pagesHoney For Nutrition and Health A ReviewHarunNo ratings yet
- The ONE by Takalani MDocument221 pagesThe ONE by Takalani MJabbie SihongoNo ratings yet
- Peroxide Value SOPDocument2 pagesPeroxide Value SOPsuresh kumarNo ratings yet