Professional Documents
Culture Documents
Chemistry 1:2:2021
Uploaded by
Eiman FahmiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry 1:2:2021
Uploaded by
Eiman FahmiCopyright:
Available Formats
2202
monday
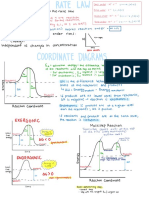
RATE OF REACTION
Definition example average rate of reaction :
rate of reaction Is the changes in the quantity
; ,%¥h%e
of the reactant per unit time or the changes § overall average rate of
in the quantity of product per unit time . E reaction
€
& total volume of gas collected
Formula
=
time taken
so t
v
}
- '
cm
f
rate of change in the quantity of reactant product a
reaction time taken for the change to occur
average rate of reaction for the
E
/
first t seconds
- - -
•
during the reaction :
quantity of reactant Ma EE total volume of gas collected in
&
Bo e the first t -
, seconds
5
2
quantity of product ti
time taken
-
O '
\
cm35
Unit for rate of reaction t, -
O
Vi I
cm3g
-
' "
95 9 minute 3 t,
mass or
" "
"
cm 's or cm
'
volume minute z
Vz
moldm's
'
moldm minute
' " u
concentration the average rate front , tot ,
-
or ,
EV '
total volume of gas collected
&
Determine the rate of reaction E from ti tot .
°
ti ta .
time taken
must be made based on the changes that are z -
Vi
cm 's
- i
observable and can be measured in a certain ta t ,
-
period of time .
nstantenous rate of reaction :
observable changes : rate of reaction at a particular point of time
determine by plotting a graph
formation of precipitate
2) decrease in the mass of reactants method :
3) increase in volume of gases
4) Changes in pressure draw a tangent to the curve
§
5) changes in electrical conductivity §
at time t .
6) changes in pH value E
B
E
3
° t
Average rate of reaction and instantaneous
rate of reaction 2 use the tangent to complete a
vertically angled triangle -
÷!Eo÷÷÷i
2 rate of reaction
average
instantaneous the triangle can be drawn in
various sizes .
the bigger the
average rate of reaction : triangle ,
the more accurate
average value for the rate of reaction that it can be to determine the
occurs in a particular time interval .
tangent gradient .
3
calculate the gradient of the tangent to the curve
§ V2 - - - - - - - -
;÷i÷÷
rate of reaction gradient of the
°
EE
I
,
at time t tangent attimet
-
50
t t tz
,
V
t
Vz VI
3g I
- -
-
(m
tz -
t ,
* extra
method to draw the for reaction that Involve
£¥i
ios tangent gradient
-
a decrease in the total mass
E - of the reactants
E i
i
'
O
t
You might also like
- Making Ammonia: WorksheetDocument4 pagesMaking Ammonia: WorksheetckNo ratings yet
- Materials For Civil and Construction Eng PDFDocument5 pagesMaterials For Civil and Construction Eng PDFOswaldo AcchoNo ratings yet
- Astm A 123 17 PDFDocument9 pagesAstm A 123 17 PDFVu Ba100% (2)
- Hot Weather Concreting CTIFDocument5 pagesHot Weather Concreting CTIFGang GaNo ratings yet
- History of Diphenylamine TestDocument2 pagesHistory of Diphenylamine Testkenneth VonNo ratings yet
- Supercritical Fluid Technology For Drug Product Development (2004)Document688 pagesSupercritical Fluid Technology For Drug Product Development (2004)Regiani Almeida Rezende100% (1)
- Brochure Retrofits 2024 2Document5 pagesBrochure Retrofits 2024 2Ale GuerraNo ratings yet
- 統計學期中考 2Document1 page統計學期中考 2玟筑黃No ratings yet
- 071 CLU3 Rev-5.0Document17 pages071 CLU3 Rev-5.0Piotr StankiewiczNo ratings yet
- Data SheetDocument9 pagesData SheetMahmod DsokyNo ratings yet
- Change Impact Analysis TemplateDocument5 pagesChange Impact Analysis TemplatesreekantthNo ratings yet
- Hydrostatics 2.a ExercisesDocument3 pagesHydrostatics 2.a ExercisesKat DjordjevicNo ratings yet
- Chemical KineticsDocument2 pagesChemical KineticsMelvin SotoNo ratings yet
- Recognizing Expenses When Incurred Rather Than When PaidDocument2 pagesRecognizing Expenses When Incurred Rather Than When PaidNath BongalonNo ratings yet
- Evonik-Tomadol (Materia Prima)Document1 pageEvonik-Tomadol (Materia Prima)Ana Isabel TriviñoNo ratings yet
- MMPI2 RFOverview Mini MNL HQDocument11 pagesMMPI2 RFOverview Mini MNL HQMaham Fatima0% (1)
- O J T Check SheetDocument1 pageO J T Check SheetabhishekNo ratings yet
- Operator'S Manual: 18 Volt Battery ChargerDocument20 pagesOperator'S Manual: 18 Volt Battery ChargerFrancisco GonzalezNo ratings yet
- Inst Intl 1Document24 pagesInst Intl 1Josito SegoviaNo ratings yet
- Chemistry For EOR (Miscible Displacement)Document49 pagesChemistry For EOR (Miscible Displacement)AliNo ratings yet
- PPMP FormDocument2 pagesPPMP Formrochelle lucilo- de VeraNo ratings yet
- Study Stone Durability 2019Document4 pagesStudy Stone Durability 2019mz zhenNo ratings yet
- Electric CurrentDocument1 pageElectric Currentsarthakyedlawar04No ratings yet
- MWI of Oil Sample Ver 0Document3 pagesMWI of Oil Sample Ver 0Haitham YoussefNo ratings yet
- Tugas DR Lintang SklofosfamidDocument10 pagesTugas DR Lintang Sklofosfamidbaim muachNo ratings yet
- Estudio Filtro Grasa Raising The BarDocument16 pagesEstudio Filtro Grasa Raising The BarOscar Barón GaonaNo ratings yet
- 2010 Syll 9696 Paper 1 Physical Core Unit 2 Atmosphere and WeatDocument10 pages2010 Syll 9696 Paper 1 Physical Core Unit 2 Atmosphere and WeatkatsandeNo ratings yet
- Current ElectricityDocument74 pagesCurrent ElectricitymahiNo ratings yet
- Electric CurrentDocument1 pageElectric CurrentMahes JeyNo ratings yet
- Preparation of AlkensDocument2 pagesPreparation of AlkensNaboth MpeirweNo ratings yet
- Ui CuroxDocument5 pagesUi CuroxHuber AlvaradoNo ratings yet
- Time Log Sheet - AmazingExcel AcademyDocument1 pageTime Log Sheet - AmazingExcel AcademyLeny HrNo ratings yet
- POST Newspaper For 27th of June, 2015Document88 pagesPOST Newspaper For 27th of June, 2015POST NewspapersNo ratings yet
- B1AIP30 - ProjectplanDocument21 pagesB1AIP30 - ProjectplanArwin SomoNo ratings yet
- Exam II Practice ProblemsDocument8 pagesExam II Practice ProblemsReshamNo ratings yet
- GHF - S of TT - Non Tech CurriculumDocument12 pagesGHF - S of TT - Non Tech CurriculumDxmplesNo ratings yet
- SeparationDocument2 pagesSeparationpnwfh7qx78No ratings yet
- Purchase: 30 Days 1 Months ForecastDocument3 pagesPurchase: 30 Days 1 Months ForecastLucky ParasharNo ratings yet
- Trabajo de Cementacion ExitosaDocument15 pagesTrabajo de Cementacion Exitosajoel linneoNo ratings yet
- Applied Macanics and McaDocument2 pagesApplied Macanics and Mcaaniketkumarverma475No ratings yet
- I Am Sharing 'DTR AQuibal AUGUST 9' With YouDocument23 pagesI Am Sharing 'DTR AQuibal AUGUST 9' With YouAlvin QuibalNo ratings yet
- Philips 1009572dbDocument4 pagesPhilips 1009572dbRumyana AvramovaNo ratings yet
- 2015 PPMP FormatDocument2 pages2015 PPMP FormatNiel JohnNo ratings yet
- Mtto de Ups Preventivo Check ListDocument1 pageMtto de Ups Preventivo Check ListCristihanNo ratings yet
- YO TE DIRÉ (Bolero)Document2 pagesYO TE DIRÉ (Bolero)RaymondJongNo ratings yet
- Section 2 - Downhole Tool SystemsDocument38 pagesSection 2 - Downhole Tool SystemsAlejandro ChavarriaNo ratings yet
- Stack Height DeterminationDocument3 pagesStack Height DeterminationJaydeep KapadiaNo ratings yet
- Mthhyep: Tyz Az÷ Tyz, N÷ Tyz A°Document1 pageMthhyep: Tyz Az÷ Tyz, N÷ Tyz A°Eswara ReddyNo ratings yet
- Gen & Admin-Staff SalariesDocument3 pagesGen & Admin-Staff SalariesAsim JavedNo ratings yet
- Incidence Map 09202021Document1 pageIncidence Map 09202021haeli spearsNo ratings yet
- TCD 2013 EngineDocument36 pagesTCD 2013 EngineFeroz GullNo ratings yet
- 2nd Edition BrochureDocument16 pages2nd Edition Brochurepeperudy100% (1)
- Astm D 1298-12b SG HidrometerDocument8 pagesAstm D 1298-12b SG HidrometerYunizar PutriNo ratings yet
- D3e802f8707!1!9l Pumpe Duse Tdi Bew and BRMDocument24 pagesD3e802f8707!1!9l Pumpe Duse Tdi Bew and BRMJan HavelNo ratings yet
- Treatment of Sewage (Summary)Document1 pageTreatment of Sewage (Summary)Travis Tan C YNo ratings yet
- Flexible Polyurethane Foams: Product OverviewDocument2 pagesFlexible Polyurethane Foams: Product OverviewSergio Marcos KettmayerNo ratings yet
- Resin Solutions: 3formDocument2 pagesResin Solutions: 3formheshamNo ratings yet
- Skin Rejuvenation Voyager 3Document6 pagesSkin Rejuvenation Voyager 377yr72cdh6No ratings yet
- Adele's Laughing Song - Horn in F PDFDocument1 pageAdele's Laughing Song - Horn in F PDFMieczysław SmydaNo ratings yet
- Sum of Pressure Loss WorksheetDocument4 pagesSum of Pressure Loss WorksheetAnonymous jvaG8m7No ratings yet
- Scatter Radiation Exposure During Mobile X Ray ExaminationsDocument6 pagesScatter Radiation Exposure During Mobile X Ray ExaminationsMauro Rojas ZúñigaNo ratings yet
- Phy 20-24 1st Sem MDocument2 pagesPhy 20-24 1st Sem MMadni BhuttaNo ratings yet
- Halogenoalkanes TestDocument4 pagesHalogenoalkanes TestDr.CharinNo ratings yet
- A Stopped-Flow Kinetics Experiment For Advanced Undergraduate Laboratories: Formation of Iron (III) ThiocyanateDocument4 pagesA Stopped-Flow Kinetics Experiment For Advanced Undergraduate Laboratories: Formation of Iron (III) ThiocyanateJuraj AhelNo ratings yet
- Enzymatic Activity Levels Vs Temperature Lab ReportDocument9 pagesEnzymatic Activity Levels Vs Temperature Lab Reportapi-387603251100% (1)
- JAMB Syllabus For ChemistryDocument22 pagesJAMB Syllabus For ChemistryOluebube Uchenna100% (1)
- Alkenes 2Document45 pagesAlkenes 2cikgu_amin100% (1)
- Chemistry ConceptsDocument60 pagesChemistry ConceptsElla Jean LanoteNo ratings yet
- CLS-Science ELS Workbook 8Document5 pagesCLS-Science ELS Workbook 8lsavaglia1990No ratings yet
- Snappyhexmesh Tutorial: Shot Sleeve For High Pressure Die CastingDocument16 pagesSnappyhexmesh Tutorial: Shot Sleeve For High Pressure Die CastingSukhamMichaelNo ratings yet
- Derivatization Reactions and Reagents For GC AnalysisDocument28 pagesDerivatization Reactions and Reagents For GC Analysisfarkad rawiNo ratings yet
- Strategic Intervention Material in Chemical ReactionsDocument15 pagesStrategic Intervention Material in Chemical ReactionsLorna AggabaoNo ratings yet
- AP Biology Chapter 8 HomeworkDocument3 pagesAP Biology Chapter 8 HomeworkViktor NaminskyNo ratings yet
- Chemistry Assignment Chapter 1 Chemical ReactionsDocument3 pagesChemistry Assignment Chapter 1 Chemical ReactionsRuchi Jain100% (1)
- p1541 - Vol 84 - 9 PDFDocument5 pagesp1541 - Vol 84 - 9 PDFmresearchNo ratings yet
- AQA Bioenergetics Knowledge OrganiserDocument2 pagesAQA Bioenergetics Knowledge OrganiserDan LiNo ratings yet
- Unit 3Document16 pagesUnit 3Himanshu SinghNo ratings yet
- Elimination Rxn'sDocument72 pagesElimination Rxn'sblackz0idNo ratings yet
- Formaldehyde From MethanolDocument6 pagesFormaldehyde From MethanolAleem AhmedNo ratings yet
- HppoDocument11 pagesHpposaragineth.saNo ratings yet
- Chapter 7 Carbonyl Method of Metal Powder ProductionDocument9 pagesChapter 7 Carbonyl Method of Metal Powder ProductionUlises Quintana CarhuanchoNo ratings yet
- Preformulation Studies WhoDocument20 pagesPreformulation Studies WhoparinafernsNo ratings yet
- 10 Chem EquilibriumDocument5 pages10 Chem EquilibriumAnnie GraceNo ratings yet
- Nitric Acid MSDSDocument6 pagesNitric Acid MSDSMostafa AlakhliNo ratings yet
- HydrocarbonDocument94 pagesHydrocarbonArshNo ratings yet
- Cre2 Catalyst-2 PDFDocument82 pagesCre2 Catalyst-2 PDFSunilNo ratings yet
- General Chemistry Course OutlineDocument3 pagesGeneral Chemistry Course OutlineShairuz Caesar Briones DugayNo ratings yet
- Chapter 14, Worksheet 1 & Video Notes "Rates Chem RXNS" Name - Part I: Potential Energy DiagramsDocument2 pagesChapter 14, Worksheet 1 & Video Notes "Rates Chem RXNS" Name - Part I: Potential Energy DiagramsPhạm Bùi Quốc QuyềnNo ratings yet
- A Short Guide To Arrows in ChemistryDocument1 pageA Short Guide To Arrows in ChemistryJefferson RibeiroNo ratings yet