Professional Documents
Culture Documents
Kmme Te101 - 1
Kmme Te101 - 1
Uploaded by
ayla sözen0 ratings0% found this document useful (0 votes)
6 views6 pagesOriginal Title
KMME TE101_1
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views6 pagesKmme Te101 - 1
Kmme Te101 - 1
Uploaded by
ayla sözenCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 6
THERMODYNAMICS
Briefly, the second law of thermodynamics states that heat
naturally flows from hot to cold, or equivalently, it states that
you cannot convert all of heat to mechanical work. I explained
how these two statements are equivalent in the class.
One important result is that, in the most efficient heat engine,
the heat transfers have to take place isothermally; this was the
Carnot engine working on the Carnot cycle. There are two other
cycles having the same efficiency as the Carnot cycle we have
seen in the class: Stirling cycle and Ericsson cycle. I
explained how Carnot efficiency is obtained in these cycles using
internal heat transfer between system components, called
regeneration.
None of the engines based on these cycles is practical (they are
efficient but give so little specific work that they can barely
overcome their internal friction), and only the Stirling cycle
has seen limited applications. They mostly serve as models that
we imitate in real life cycles so that we may increase
efficiency.
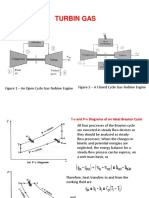
Then we saw Brayton cycle which is the basic one used in gas
turbine power plants and jet propulsion.
These idealized power cycles working on gas (air) are called air-
standard power cycles.
In Brayton cycle, heat transfer is at constant pressure, not
isothermal, therefore its efficiency is lower than that of
Carnot. So people naturally looked for ways to improve
efficiency, knowing of course that the efficiency, even
theoretically, cannot surpass Carnot efficiency.
One way to do this is called regeneration (or recuperation); we
have seen this term before. It refers to using hot gases within
the plant to heat cold gases at another location within the
system using heat exchangers.
In an actual plant, the heat exchanger at the top (where Q L is
given out-rejected) does not exist; the exhaust gases go out and
fresh air is taken into the compressor. But from thermodynamic
point of view, there is a heat exchanger.
Looking at the figure, the hot gases at the turbine exhaust are
used to heat cold gases at the entrance to the combustion chamber
(where heat transfer from outside Q H occurs). Of course, for
this to work, exhaust gases must be hotter, i.e., T 4 >T 2 in the
cycle. Otherwise no regeneration is possible.
(Thermal) efficiency
qL T −T 1
η=1− =1− A
qH T 3−T B
I remind you again that we always assume ideal gas with constant
specific heats (unless otherwise stated); and if the mass flow
rate in the cycle is ṁ , Q= ṁq and ṁ’s cancel.
The heat exchange within the regenerator does not come in here
because it is not an exchange with the surroundings (as was the
case in Stirling and Ericsson cycles).
For ideal regenerator
T B=T 4 , T A=T 2
i.e., all possible heat flow between the hot and cold gases
occurs. For example, the temperature of the hot gases decreases
to as low as the temperature of the cold gases entering the
regenerator.
This, of course is not possible. Actual points will be at T A '
and T B ' in the cycle. For the non-ideal, actual regenerator,
define regenerator efficiency as
h B ' −h2 h4 −h A '
ηreg = =
h B−h2 h4 −h A
(think why these are equal). You can replace h with T since
c p is constant.
Example.
Ideal Brayton cycle. Air k =1.4 . Temperatures T 1=21.1℃ ,
p2
T 3=982.2 ℃. Pressure ratio =8. Compute the efficiency for the
p1
following cases: no regeneration, with an ideal regenerator, and
with 70 % regenerator efficiency.
Ideal cycle, as we have seen before corona,
1
η=1− k−1
=0.448
p2
( )
p1
k
The case with ideal regenerator, T B=T 4 , T A=T 2 ,
T 2−T 1
η=1−
T 3 −T 4
k−1
p2
T 2=T 1
( )
p1
k
=533 K
T3
T 4= k−1
=693 K
p2
( )
p1
k
η=0.575
(increased).
Real regenerator with 70 % efficiency,
T A ' =T 4−ηreg ( T 4 −T 2 ) =307 ℃
T B ' =T 2+ ηreg ( T 4 −T 2 ) =372℃
T A −T 1
'
η=1− =0.468
T 3−T B '
(increased but not as much).
Another method to increase efficiency is intercooling (in the
compressor) and reheating (in the turbine). Below is a plant
with intercooling, reheating and regeneration:
(Note that this one is open to air, there is no difference as I
said).
The reason this one is more efficient is that compression and
expansion processes (zig-zags at the bottom and top) imitate the
constant temperature (isothermal) compression and expansion in
the Ericsson cycle, i.e., we are trying to approximate the
Ericsson cycle.
Think about how the T-S diagram is drawn.
Of course all these modifications to increase efficiency
increases cost.
You might also like
- ch08 41 85Document52 pagesch08 41 85Giuseppe TestarossaNo ratings yet
- Gas TurbineDocument7 pagesGas TurbineVishal ReddyNo ratings yet
- Brayton Cycle With RegenerationDocument6 pagesBrayton Cycle With RegenerationMeshal Al-mutairi100% (1)
- 07 Gas Turbine Power PlantsDocument37 pages07 Gas Turbine Power PlantsLozada JohnNo ratings yet
- City and Guilds 9210 Level 6 Module - Unit 128 Applied ThermodynamicsDocument20 pagesCity and Guilds 9210 Level 6 Module - Unit 128 Applied ThermodynamicsAlaa ShammaaNo ratings yet
- Brayton CycleDocument5 pagesBrayton CycleAnonymous yorzHjDBd100% (1)
- Lectures in Thermodynamics Claus BorgnakkeDocument38 pagesLectures in Thermodynamics Claus BorgnakkeJkun gamingNo ratings yet
- Gas Turbine Theory by Hih Saravanamuttoo, H. Cohen & GFC RogersDocument11 pagesGas Turbine Theory by Hih Saravanamuttoo, H. Cohen & GFC RogersduccNo ratings yet
- GT ThermodynamicsDocument22 pagesGT Thermodynamicsforzama100% (1)
- Chapter Five PDFDocument24 pagesChapter Five PDFعبدالله رعد حران 32No ratings yet
- CH 10Document38 pagesCH 10정민교No ratings yet
- Gas TurbinesDocument66 pagesGas Turbinessripoojitha511No ratings yet
- In This Tutorial You Will Do The Following. Revise Gas Expansions in TurbinesDocument22 pagesIn This Tutorial You Will Do The Following. Revise Gas Expansions in TurbinesPardeep bainsNo ratings yet
- Turbine: The Effect of Friction On The Joule CycleDocument13 pagesTurbine: The Effect of Friction On The Joule CycleGeorge AniborNo ratings yet
- T 9109Document9 pagesT 9109Ayush Singh BeruvarNo ratings yet
- Gas Turbine With A Free Power Turbine: The Gas Turbine and The Specific Fuel Consumption (Document5 pagesGas Turbine With A Free Power Turbine: The Gas Turbine and The Specific Fuel Consumption (surafelshimelesNo ratings yet
- Brayton Cycle Closed Cycle OnlyDocument2 pagesBrayton Cycle Closed Cycle OnlyAlvin LoocNo ratings yet
- Modifications On Brayton CyclesDocument42 pagesModifications On Brayton CyclesMohamed KhaledNo ratings yet
- Gas Power Cycle - Part 2 v2Document35 pagesGas Power Cycle - Part 2 v2Shahran IezzatNo ratings yet
- Siklus Brayton Contoh:: P P T TDocument7 pagesSiklus Brayton Contoh:: P P T TDicky PasaribuNo ratings yet
- Gas - Turbine - CycleDocument41 pagesGas - Turbine - CycleKalpaniNo ratings yet
- Gas Turbine CyclesDocument60 pagesGas Turbine Cyclesالأردني JordanianNo ratings yet
- 11 77P - DoneDocument2 pages11 77P - DonegowthamAG07No ratings yet
- Turbin Gas: Siklus Terbuka Turbine GasDocument34 pagesTurbin Gas: Siklus Terbuka Turbine GasRezky RinaldyNo ratings yet
- SPPU Sem 6 ARAC 1, 2& 3 NOTESDocument52 pagesSPPU Sem 6 ARAC 1, 2& 3 NOTESAshish RejikumarNo ratings yet
- Isentropic Process PDFDocument2 pagesIsentropic Process PDFJeebee Logroño AloNo ratings yet
- Handbook Termodinamika, Komang SuardikaDocument22 pagesHandbook Termodinamika, Komang SuardikaKomang SuardikaNo ratings yet
- In This Tutorial You Will Do The Following. Revise Gas Expansions in TurbinesDocument23 pagesIn This Tutorial You Will Do The Following. Revise Gas Expansions in TurbinesHayderr HassNo ratings yet
- Vapor Power Cycles: Thermodynamics Professor Lee CarknerDocument16 pagesVapor Power Cycles: Thermodynamics Professor Lee CarknerVaidya AarshNo ratings yet
- Thermodynamics IIDocument25 pagesThermodynamics IIpalmer okiemuteNo ratings yet
- RAC TutorialDocument30 pagesRAC Tutorialkhushal bhanderiNo ratings yet
- MPE 371 CH 1 - Air Standard Cycles-1Document27 pagesMPE 371 CH 1 - Air Standard Cycles-1Abraham MugambiNo ratings yet
- Two-Stage Gas Turbine: C K C KDocument3 pagesTwo-Stage Gas Turbine: C K C KgowthamAG07No ratings yet
- Topic 8 Gas Turbine Power PlantDocument6 pagesTopic 8 Gas Turbine Power PlantILEENVIRUSNo ratings yet
- Paper Rebal and MozherDocument6 pagesPaper Rebal and MozhermurtadaNo ratings yet
- 125452033-Thermodynamics D.J.Dunn PDFDocument185 pages125452033-Thermodynamics D.J.Dunn PDFkalite123No ratings yet
- Gas Turbine 2 - 2022Document55 pagesGas Turbine 2 - 2022Osama M. GamalNo ratings yet
- Second Law of Thermodynamics: T T Q QDocument10 pagesSecond Law of Thermodynamics: T T Q Qnellai kumarNo ratings yet
- Engineering Council Certificate Level Thermodynamic, Fluid and Process Engineering C106 Tutorial 5 - Ideal Engine CyclesDocument14 pagesEngineering Council Certificate Level Thermodynamic, Fluid and Process Engineering C106 Tutorial 5 - Ideal Engine CyclesDipeshNo ratings yet
- Bryton Cycle PDFDocument9 pagesBryton Cycle PDFEddyNo ratings yet
- Gas Steam and Combined CyclesDocument84 pagesGas Steam and Combined CyclesJane Smith100% (1)
- 6159b9a3-a853-4554-bf38-b5a65964ffceDocument39 pages6159b9a3-a853-4554-bf38-b5a65964ffceUsman AliNo ratings yet
- Gas-Turbine Cycles: 3.1 PerformanceDocument2 pagesGas-Turbine Cycles: 3.1 PerformancekouroshNo ratings yet
- 2007 - DiPippo - Ideal Thermal Efficiency For Geothermal Binary PlantsDocument10 pages2007 - DiPippo - Ideal Thermal Efficiency For Geothermal Binary PlantsAntokNo ratings yet
- 300 Kpa 300 K 100 Kpa 900 KDocument2 pages300 Kpa 300 K 100 Kpa 900 KgowthamAG07No ratings yet
- Chapter 3 Gas Turbine: 3.1 Brayton CycleDocument10 pagesChapter 3 Gas Turbine: 3.1 Brayton CycleBryan AveigaNo ratings yet
- MMAN2700ThermoProblemSheet7 - Entropy 2nd LawDocument3 pagesMMAN2700ThermoProblemSheet7 - Entropy 2nd Lawgrandw9524No ratings yet
- 0 Bahan 9 Kuliah Pe DaringDocument16 pages0 Bahan 9 Kuliah Pe Daringmutiara eva syehrinaNo ratings yet
- RefrigerationDocument163 pagesRefrigerationali105No ratings yet
- Gas TurbineDocument14 pagesGas Turbineاحمد الربيعي100% (1)
- TURBIN GasDocument15 pagesTURBIN GasMuna Alfi ANo ratings yet
- Intermediate Thermodynamics Cycles: Heat-Engine CycleDocument7 pagesIntermediate Thermodynamics Cycles: Heat-Engine Cycleabdul_rehman_124No ratings yet
- Problem With SolutionDocument3 pagesProblem With SolutionHardiNo ratings yet
- Tutorial Bell Colleman Cycle 1Document10 pagesTutorial Bell Colleman Cycle 1AbomerNo ratings yet
- School of Physics and AstronomyDocument4 pagesSchool of Physics and AstronomyzjnsrbtNo ratings yet
- Gas Cycles Otto, Diesel, Dual CyclesDocument43 pagesGas Cycles Otto, Diesel, Dual Cyclesprasad5034100% (1)
- Thermodynamics Fundamentals For Energy Conversion Systems: (Continued)Document24 pagesThermodynamics Fundamentals For Energy Conversion Systems: (Continued)MUmairWaheedNo ratings yet
- Fluid Dynamics and Turbomachines: WEEK 1: AssignmentDocument16 pagesFluid Dynamics and Turbomachines: WEEK 1: AssignmentAsrar AhmedNo ratings yet
- Acoustic Emission Analysis in Fracture MechanicsDocument28 pagesAcoustic Emission Analysis in Fracture MechanicsVYSHAKH VENUGOPALNo ratings yet
- Excel-Solution of The Implicit Colebrook Flow Friction EquationDocument14 pagesExcel-Solution of The Implicit Colebrook Flow Friction EquationLasandu WanniarachchiNo ratings yet
- Applied Hydraulics: Hydraulic PumpsDocument136 pagesApplied Hydraulics: Hydraulic Pumpshasan bishNo ratings yet
- The American University in Cairo Fundamentals of Fluid MechanicsDocument7 pagesThe American University in Cairo Fundamentals of Fluid MechanicsKarim KhalafNo ratings yet
- U Shape SpringDocument7 pagesU Shape SpringBastian AguilarNo ratings yet
- Is 3370 2 2009Document18 pagesIs 3370 2 2009Manish AhujaNo ratings yet
- Tema 1. Ciclo OttoDocument19 pagesTema 1. Ciclo Ottojesus matus carmonaNo ratings yet
- 1008 2294 PDFDocument17 pages1008 2294 PDFالاسمر الجنوبيNo ratings yet
- Chapter 10, Problem 8CDocument9 pagesChapter 10, Problem 8CJohnNo ratings yet
- New Directions in Race Car Aerodynamics by Joseph Katz - Excerpt From Chapter 6: Aerodynamics of The Complete VehicleDocument15 pagesNew Directions in Race Car Aerodynamics by Joseph Katz - Excerpt From Chapter 6: Aerodynamics of The Complete VehicleBentley Publishers100% (1)
- UU Triaxial Test - Lab ManualDocument6 pagesUU Triaxial Test - Lab ManualmmNo ratings yet
- Mechanical Systems and Signal ProcessingDocument23 pagesMechanical Systems and Signal ProcessingBeatriz WangNo ratings yet
- 8.thermo Gases and Vapour Mixtures Obj by RajputDocument3 pages8.thermo Gases and Vapour Mixtures Obj by RajputMuruganantham MajesticNo ratings yet
- AUTODYN - Chapter 7 - ALE - SolverDocument9 pagesAUTODYN - Chapter 7 - ALE - SolverFabiano OliveiraNo ratings yet
- Ashrae 90.1 PDFDocument8 pagesAshrae 90.1 PDFmohamadNo ratings yet
- Splice 03Document15 pagesSplice 03LexNo ratings yet
- Rules of MixtureDocument33 pagesRules of MixtureDohyun KimNo ratings yet
- 4 - 5.1 - Fluid Action On Surfaces (Plane Surface)Document16 pages4 - 5.1 - Fluid Action On Surfaces (Plane Surface)Jacky CagampanNo ratings yet
- Physics112 Fall2018 HW 1bDocument7 pagesPhysics112 Fall2018 HW 1bJose DavidNo ratings yet
- Eesy Solutions: Engineering Equation Solver NewsletterDocument8 pagesEesy Solutions: Engineering Equation Solver NewsletterFarman AliNo ratings yet
- Models - Mph.rock Fracture FlowDocument8 pagesModels - Mph.rock Fracture FlowAl-Kawthari As-SunniNo ratings yet
- Prestressed and Precast Concrete: Chapter 4: Prestress LossesDocument24 pagesPrestressed and Precast Concrete: Chapter 4: Prestress LossesKhalilRoumaniNo ratings yet
- Lecture # 02 Goverening Equations and Its DiscritizationDocument55 pagesLecture # 02 Goverening Equations and Its Discritizationmaheshj90No ratings yet
- FEA 16 Mark QBDocument12 pagesFEA 16 Mark QBMr. N. Aravindkumar Asst Prof MECHNo ratings yet
- Experiment No: 5: Measurement of Co-Efficient of Discharge For An Orifice MeterDocument7 pagesExperiment No: 5: Measurement of Co-Efficient of Discharge For An Orifice MeterManoj guptaNo ratings yet
- Thermodynamic Analysis of Compressed Air VehicleDocument12 pagesThermodynamic Analysis of Compressed Air VehicleDiditEkaPrasetyaNo ratings yet
- Continuum Mechanica Cheat SheetDocument5 pagesContinuum Mechanica Cheat SheetAli100% (1)
- AWS100 Appendix 12 FatigueDocument72 pagesAWS100 Appendix 12 FatigueHector TixeNo ratings yet