Professional Documents
Culture Documents
Study of TSP Fertilizer Production & Quality
Uploaded by
ZeroRecoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Study of TSP Fertilizer Production & Quality
Uploaded by
ZeroRecoCopyright:
Available Formats
Journal of Science and Arts Year 17, No. 4(41), pp.
779-788, 2017
ORIGINAL PAPER
STUDY OF TSP FERTILIZER
PRODUCTION & QUALITY
N. CHAOUQI 1,2, M. BOUZZIRI1, M. EL GHAROUS 3
_________________________________________________
Manuscript received: 10.09.2017; Accepted paper: 04.11.2017;
Published online: 30.12.2017.
Abstract. Results obtained showed that some essential manufacturing factors needs to
be respected throughout the production loop. These factors will help obtaining a slurry exits
with the desired chemical characteristics of P 2 O 5SE (33%), Al (10%), P 2 O 5SE +Citrate
(34.5%), H 2 O (22%), P 2 O 5total (39%)). It is necessary to keep a temperature within 100 ± 5 °
C., with a vapor pressure of 6 to 7 bars, an average density of H 3 PO 4 (1470 g/L) and the
solid rate in the feed acid less than 2%. After the reaction phase, the slurry is conveyed to the
granulator where it is mixed with the recycled product at a recycling rate (RT) close to 3 and
a K ratio between 0.1 and 0.2. This parameter is very important to be monitored to ensure a
Good penetration of heat into the next phase in a relatively short time. During the drying
process, the product humidity at the outlet must be in the range of 3.5% to 5% to allow the
reaction to continue during the developing period. At the exit of the drying tube, the product
is in very variable grains diameter (D), the objective of the following step is to extract the
grain size range between 2 and 4 mm. To do so we changed the mesh size of the lower webs to
3/50 and 3.2 / 30 mm, which reduces the quantity of particle size less than 2%. Thus, the
particles of diameter more than 3.15 mm must be continuously controlled to reach 64%. This
portion will ensure conformity of the product for D50 and for the two intervals [2 – 3.15 mm]
and [3.15 - 4 mm]. In the Storage Hall the reaction continues, the unconverted phosphate will
be attacked by the unreacted H 3 PO 4 , and according to a monitoring of the evolution of the
chemical composition of the final product (TSP). The required ripening time is limited to 21
days.
Keywords: TSP fertilizers, quality, granulation, drying.
1. INTRODUCTION
Phosphorus is one of the most essential elements for plant growth after nitrogen [1].
Phosphorus plays an important role in many physiological and biochemical plant activities,
one of the advantages of feeding plants with phosphorus is to create deeper and more
abundant roots [2, 3]. Phosphorus causes early maturation in plants, decreases grain mold,
increases leaf chlorophyll content and may positively affect photosynthesis, thus improving
crop quality [4, 5]. However, the availability of this nutrient for plants is limited by different
chemical reactions (formation of strong links between phosphorus with Ca2 + and Mg2+ in
alkaline pH and the same links with Fe2 + and Al3 + in acid soils [1, 6, 7]. Thus, by certain
agricultural practices such as the excessive application of chemical phosphate fertilizer, a
large proportion of phosphorus in chemical fertilizer becomes unavailable for plants after its
1
FST-University Hassan 1st, Laboratory of Applied Chemistry and Environment, Settat, Morocco.
E-mail: chaouqinora@live.fr.
2
Office Chérifien des Phosphates (OCP), Service Engrais, Division Maroc-Chimie, Safi, Morocco.
3
University Mohammed VI Polytechnique, Benguerir, Morocco.
ISSN: 1844 – 9581 Chemistry Section
780 Study of TSP fertilizer… N. Chaouqi et al.
application in the soil [8-13]. When phosphate fertilizers are not used properly, in addition to
economic loss, leads to soil degradation. Therefore, better management of the fertilizer
industry can be very effective in eliminating this possibility (FNCUMA, 1996). Thus, to
improve the efficiency of phosphate fertilizers, they must be well distributed in the soil in
order to increase the chances of contact with the roots (the 4R Stewardship, Decision-Making-
Guide-Phosphorus, by IPNI-USA, 2017) [13].
A critical analysis of the TSP production chain was initiated in the Maroc Chimie
division, OCP-Safi, its objective is to increase the overall performance of the cropping
systems by providing a balanced P fertilization which gives an optimal economic return. This
objective, can be achieved only if the operating conditions are established strictly to
AFCOME specifics, controlling the process step parameters, and making a chemical analysis
of finished product.
2. MATERIALS AND METHODS
2.1. THE TSP PRODUCTION PROCESS
The production of TSP fertilizers according to the Saint-Gobain process (Fig. 1)
involves attacking the phosphate with phosphoric acid (42% P 2 O 5 ).
The objective of this attack is to obtain mono-calcium, monohydrate phosphate soluble
in water and therefore directly absorbed by plants, according to the overall reaction:
2Ca 3 (PO 4 ) 2 + 5H 3 PO 4 + 9H 2 O 3Ca (H 2 PO 4 ) 2 .H 2 O + 3Ca (HPO 4 )·2H 2 O
Figure 1. TSP production process.
www.josa.ro Chemistry Section
Study of TSP fertilizer… N. Chaouqi et al. 781
2.2. ANALYTICAL METHODS USED FOR CONTROL OF THE MODIFIED PARAMETERS:
2.2.1. Humidity measurement: According to AFNOR 11-464.
2.2.2. % P2O5SE&SC & % of acid not reacted (AL): According to the Physicochemical Laboratory
Analysis of the ‘Division Maroc Chimie (OCP-Safi)’ ME00-PSC-2-ICS / P / C / L.
3.RESULTS AND DISCUSSION
3.1. REACTION
Mass flow rate of the feed tank:
𝐐𝐏𝟐 𝐎𝟓 (𝐇𝟑 𝐏𝐎𝟒 ) + 𝐐𝐏𝟐 𝐎𝟓 (𝐩𝐡𝐨𝐬) + 𝐐𝐭(𝐯𝐚𝐩) = 𝐐𝐏𝟐 𝐎𝟓 (𝐛) + 𝐐𝐭(𝐠𝐚𝐳)
Partial flow (relative to P 2 O 5 ):
𝒙𝑷𝟐 𝑶𝟓 (𝒑𝒉𝒐𝒔) 𝑸𝒆𝑷𝟐 𝑶𝟓 (𝒑𝒉𝒐𝒔) + 𝒙𝑷𝟐 𝑶𝟓 (𝐇𝟑 𝐏𝐎𝟒 ) 𝑸𝑷𝒆 𝟐𝑶𝟓 (𝐇𝟑 𝐏𝐎𝟒 )
= 𝒙𝑷𝟐 𝑶𝟓 (𝒃) 𝑸𝒔𝑷𝟐 𝑶𝟓(𝒃) + 𝒙𝑷𝟐 𝑶𝟓 (𝒈𝒂𝒛) 𝑸𝒔(𝒈𝒂𝒛)
In order for slurry to obtain the chemical characteristics P 2 O 5SE (33%), AL (10%),
P 2 O 5SE+Citrate (34.5%), H 2 O (22%), P 2 O 5 total (39%), for a flow rate, Q (P 2 O 5TSP ) = 27.3 t / h
and a slurry mass of 0.075t/t TSP , several parameters are taken into consideration:
3.1.1. The particle size of phosphate feed
Analyzes show that ensuring a phosphate particle size of 90% of the past with a 160
μm sieve increases the attack surface, the chemical reaction is easier as the surface area
offered to reagents is larger, requiring shorter period and less acid to be attacked.
3.1.2. Acidulation rate
𝑸𝑷𝟐 𝑶𝟓 (𝐇𝟑 𝐏𝐎𝟒 )
𝑻=
𝑸𝑷𝟐 𝑶𝟓 (𝒑𝒉𝒐𝒔)
2H 3 PO 4 P 2 O 5 + 3H 2 O
Ca 3 (PO 4 ) 2 P 2 O 5 + 3CaO
This ratio is defined as the amount in grams of P 2 O 5 supplied by the acid required for
the attack of 100 g of phosphate. Theoretically, T = 2.
Practically, this ratio varies between 2.4 and 2.6, the impurities brought by the raw
materials, consume more acid.
CaCO 3 + H 2 O + 2H 3 PO 4 Ca (H 2 PO 4 ) 2 , H 2 O + CO 2 + H 2 O
There is a consumption of H 3 PO 4 to give slightly or not soluble phosphates.
Phosphoric acid was retrograded. Also, as the carbonate content increases, the hardness and
density of TSP increases, this causes granulation difficulties.
ISSN: 1844 – 9581 Chemistry Section
782 Study of TSP fertilizer… N. Chaouqi et al.
Al 2 O 3 + 2H 3 PO 4 → 2AlPO 4, H 2 O + H 2 O
Fe 2 O 3 + 2H 3 PO 4 → 2FePO 4, H 2 O + H 2 O
The crystalline forms of Al and Fe are insoluble in phosphoric acid, hence an increase
in the level of solid in phosphoric acid.
When the granulated phosphate rock is attacked with ortho-phosphoric acid (42%),
there will theoretically be the production of phosphate mono-calcium called the triple super
phosphate (TSP). In reality, this reaction is no longer complete for reasons of reagent
characteristics.
The impurities present in phosphoric acid and in phosphate (Often the same quality
delivered, presents an unstable profile), lead to reactions which are far from simple; they
consume acid without recovering the product in the form of additional soluble P 2 O 5 .
But these two parameters depend not only on the fertilizer service, the other services
require a particle size of 80 μm. As well as, for the quality of the feed material, for the H 3 PO 4
production service: Fe 2 O 3 has an important effect on the viscosity of the slurry. Al 2 O 3 has a
positive effect because it binds with the F- and thus eliminates the negative effect of it as a
highly corrosive agent. It also improves the crystallization allowing a regularity of growth of
the crystals in all directions of the space. CaO has a positive impact on productivity, reacts
with silica (non-reactive) and reduces its impact on the progress of production.
3.1.3. Temperature (T°C)
The temperature of the tank must be between 100 and 105 °C. If T > 105 °C this causes
a bad flow of the slurry and then a bad granulation, and if T <100 °C Gets a bad attack. The
control is made by the steam flow through the control panel. The TSP production room uses
steam about 3 to 4 t/ hour per line with a pressure of 6 to 7 bar and a temperature of 180 to
220 °C. The rate of steam flow is regulated by a regulating valve before injection into the
steam tank using steam injectors of 4 to 6 holes.
3.1.4. Density (d)
To make a slurry as smooth as possible with the desired quality, a density of H 3 PO 4 of
1480 must be ensured. A lower value (excess dilution water) causes a decrease in the
temperature of the tank and the slurry becomes too fluid, the flow of phosphoric acid must be
lowered. If d > 1480 (inadequate dilution water) leads to a dusty circuit, with deregulation of
titers, in this case it is necessary to check the condition of the densimeter.
3.1.5. Solid content (T. S)
3CaH 4 (PO 4 ) 2 + 3H 2 SO 4 + 6H 2 O 3CaSO 4 .2H 2 O + 6H 3 PO 4
The presence of H 2 SO 4 in the reaction mixture causes a decrease in the percentage of
mono-calcium phosphate which affects the composition of the TSP, in fact a H 2 SO 4 > 18 g/l
ratio leads to a decrease of P 2 O 5SE . Thereafter the rate of solid (T.S > 2%) produces an
influence on the composition (total P 2 O 5 ) of TSP. A high solids content cause a consuming
difficulties of acid, acid impurities react with other species during the reaction decreasing the
amount of P 2 O 5total .
www.josa.ro Chemistry Section
Study of TSP fertilizer… N. Chaouqi et al. 783
3.2. GRANULATION
The mass balance:
𝒈 𝒔𝒈
𝑸𝒆(𝒃) + 𝑸𝒓(𝑻𝑺𝑷) = 𝑸(𝑻𝑺𝑷) + 𝑸(𝒈𝒂𝒛)
The partial balance relative to P 2 O 5 :
𝒈 𝒈 𝒔𝒈 𝒔𝒈
𝒙𝑷𝟐 𝑶𝟓 (𝒃) 𝑸𝒆(𝒃) + 𝑹𝒓 𝒙𝑷𝟐 𝑶𝟓 (𝑻𝑺𝑷) 𝑸𝒕(𝑻𝑺𝑷) = 𝒙𝑷𝟐 𝑶𝟓 (𝑻𝑺𝑷) 𝑸(𝑻𝑺𝑷) + 𝒙𝑷𝟐 𝑶𝟓 (𝒈𝒂𝒛) 𝑸(𝒈𝒂𝒛)
The flow rate of the granulated TSP :
𝒔𝒈 𝒔𝒈
𝒈
𝒙𝑷𝟐 𝑶𝟓 (𝒃) 𝑸𝒆(𝒃) + 𝑹𝒓 𝒙𝑷𝟐 𝑶𝟓 (𝑻𝑺𝑷) 𝑸𝒕(𝑻𝑺𝑷) − 𝒙𝑷𝟐 𝑶𝟓 (𝒈𝒂𝒛) 𝑸(𝒈𝒂𝒛)
𝑸(𝑻𝑺𝑷) = 𝒈
𝒙𝑷𝟐 𝑶𝟓 (𝑻𝑺𝑷)
Ensuring a constant production rate is mainly based on a recycling rate constant,
which depends to finished product quantity.
𝑹𝒆𝒄𝒚𝒄𝒍𝒆𝒅 𝒑𝒓𝒐𝒅𝒖𝒄𝒕 𝑸𝒓(𝑻𝑺𝑷)
𝑹𝒓 = = =𝟑
𝑭𝒊𝒏𝒊𝒔𝒉𝒆𝒅 𝒑𝒓𝒐𝒅𝒖𝒄𝒕 𝑸𝒕(𝑻𝑺𝑷)
The recycling rate is between 180 and 220 T / h with a maximum capacity of 350 T /
h. The product of recycling constitutes the support of granulation phase, which is in function
of flow rate and slurry humidity. The granulation of the product requires in the granulator a K
ratio between 0.1 and 0.2.
- If K < 0.1 the efficiency of the granulator decreases causes a dusty product.
- If K> 0.2 the granulator requires frequent stops for cleaning.
Thus, the temperature of the recycled product must be carefully controlled, to ensure a value
enter 70 and 76°C.
3.3. DRYING
The main drying parameters having an influence on the physicochemical quality of the
finished product are:
3.3.1. Product temperature
The drying temperature is limited by the melting point of the fertilizer (melting point:
190°C & decomposition temperature: 240 °C (according to the TSP FDS)), the product is
admitted to the dryer at a temperature close to 85 °C. It is heated to just reach the temperature
for which there is sufficient evaporation of water.
3.3.2. Dried product humidity
The TSP leaving the dryer with a humidity about 5%. The product is dried at
approximately 5% to allow the further reaction between the free acid and the still unreacted
phosphate to continue; this reaction cannot continue if the humidity is less than 3.5%.
ISSN: 1844 – 9581 Chemistry Section
784 Study of TSP fertilizer… N. Chaouqi et al.
3.3.3. Drying time
Depends mainly on the speed and inclination of the dryer tube. In fact, for a good
penetration of tempture in grains in a relatively short time, the recorded rotation speed is: 3.5
r/min for a maximum capacity of: 350 T / h, with a slope of: 3 %.
3.4. SIEVING
At the exit of drying tube,the grains diameter is very variable. The objectif of this
operation is to extract from the product the grain size between 2 and 4 mm. The large
particles, after crushing grinding, are recycled as well as fines to granulator. The change in
sizing of 2/50 and 2.5/30 mm by 3/50 and 3.2/30 mm of the sieves reduces the fines passing
quantity through the finished product,which allowed to enter in the interval [1 -2]. The two
figures below summarize the most profitable results from various tests carried out at this
point. (The results displayed are those most adopted in the following months).
Figure 2. The changing size of sieves (LN/C2).
Figure 3. The changing size of sieves (LN/C4).
In order to have a cut of 4 mm, the corresponding opening must be between 4.4 and
4.8 at the top sieve and a cut of 2 mm its corresponding opening between 2.2 and 2.4 mm for
the lower sieve. It can be said that the efficiency of the two upper sieves in this case is
satisfactory with more than 90%.
www.josa.ro Chemistry Section
Study of TSP fertilizer… N. Chaouqi et al. 785
Figure 4. Graphical presentation of North Line before the change (A) and after (B) in [1 -2].
L.S and L.I: Tolerances of AFCOME in this interval.
The requirements of AFCOME appear in the interval [1-2], this is reflected by the
change of the meshes dimensioning of the lower webs. There is also a progression for the
interval [4-5] due to the minimization of the mesh surfaces of the upper canvases of the
sieves.
Figure 5. Presentation of the compliance rate according to AFCOME at the North line.
Figure 6. Presentation of the T.C compliance rate according to AFCOME at the South line.
These graphs result in the change of the T.C of the months before (December and
January) and after (February, March, and April) the change of the canvases. A remarkable
evolution exceeds 70% compliance at the interval [1-2] and median diameter (d 50 =Zn
(50−Cn)
+(Cn+1−Cn) x (Zn + 1 − Zn)) for the two production lines. Also for grains portions entering
the interval [4-5].
Table 1. The effect of the proportion > 3.15 on product conformity (LS).
(1conform, 0 not conform)
[3,15-4] [2-3,15] D 50 > 3,15 [3,15-4] [2-3,15] D 50 > 3,15
1 1 1 72 0 0 1 50
1 1 1 67 0 0 1 59
1 1 1 64 0 0 1 43
1 1 1 69 0 0 0 34
1 1 1 63 0 1 1 56
0 0 1 51
ISSN: 1844 – 9581 Chemistry Section
786 Study of TSP fertilizer… N. Chaouqi et al.
Figure 7. The evolution of the D50 f (3.15).
According to particle size analyzes results, the grains proportion with an average value
of 64% of 3.15 cm diameter, is that which influences the conformity of product in the two
intervals [3.15-4], [2-3.15]. These modifications are considered to be beneficial at the Storage
Hall, namely a remarkable reduction in the refusal with 1.86%.
3.5. STORAGE
In the storage hall the reaction continues, the unreacted phosphate will be attacked by
the unreacted phosphoric acid, to reach the final stage of this reaction and meet both the
commercial specifications (AFCOME Requirements), A storage time is required for the
product, known by the ripening time. Determining of ripening time, is do it by evolution of
chemical composition of TSP product (FP-TSP), namely P 2 O 5SE , P 2 O 5SE+ SC , total P 2 O 5 ,
%AL and product humidity.
Table 2. Evolution of the chemical composition Output-Line.
Days % P 2 O 5SE % P 2 O 5SE+SC % P 2 O 5total % AL % H2O
1 40,5 43,7 46,1 5,7 5.8
3 40,8 44,4 46,2 2,8 5.3
5 40,9 44,5 46,7 2,1 5.1
7 41,1 44,7 46,6 1,9 5
10 40,9 44,7 46,7 1,6 5.1
14 41,3 44,7 47,2 1,5 5
16 41,8 45,4 47,7 1,4 3.7
19 41,8 45,8 47,7 1,4 3.4
*21 41,8 45,7 47,7 1,4 3.2
22 41,8 45,7 47,7 1,4 3.2
23...29 41,8 45,7 47,7 1,4 3.2
www.josa.ro Chemistry Section
Study of TSP fertilizer… N. Chaouqi et al. 787
Table 3. Particle size of FP-TSP in the Hall.
>5 >4 >3,15 >2,5 >2 >1 Days in
mm mm mm mm mm mm the hall
0 7 41 88 97 100
Line Output
0 7 40 88 97 100
0 6 38 86 96 100
7 Days
0 5 35 84 95 100
0 6 43 88 97 100
12 Days
0 8 37 86 97 100
0 8 44 90 98 100
18 Days
1 6 41 87 96 100
0 8 37 86 97 100 21 Days
From the results obtained (Table 2) it can be said that the time required to satisfy the
AFCOME requirements is 21 days. From the physical analysis (Table 3), it can be seen that
the thermal cycle in the Storage Hall (REX1) does not influence the physical quality of the
TSP during this ripening period.
The presentation of the compliance rate of finished product TSP during the months of
February, March and April, at the level of the two storage halls:
87% 87%
73.00%
54%
38%
23.00%
South Line
15.00%
D2 0.00%
[1--2[ [2--3,15[ [3,15--4[ [4--5[
Figure 8. Comparison of the compliance rate between South Line & Hall-D 2 output.
North Line
69.23%
D1
38% 38% 36.36%
27% 36%
18.00%
0.00%
[1--2[ [2--3,15[ [3,15--4[ [4--5[
Figure 9: Comparison of the compliance rate between North Line & Hall-D1 output.
There is a remarkable drop in conformity of line to the destocked, this quality
degradation due first of all to the mixing of conforming quality production days with others
not in conformity. This mixture affects not only the physical quality but also the chemical. To
avoid this risk, we have established the FIFO strategy; the following lot will not be started
until the previous lot has been exhausted.
ISSN: 1844 – 9581 Chemistry Section
788 Study of TSP fertilizer… N. Chaouqi et al.
4. CONCLUSIONS
The production of TSP requires phosphoric acid (H 3 PO 4 ), and treated rock phosphate
containing respectively 42% and 30% of P 2 O 5 . In order for the TSP fertilizer to meet norms
of AFCOME specifics, it needs to have a minimum chemical content of 47% P 2 O 5total , 42%
P 2 O 5SE , 46%P 2 O 5SE+Citrate , and a maximum of 22 ppm Cd and 2% Al. Also, characteristics in
terms of particle size 1to 2mm (1- 4%), 2 to 3.15 mm (24-36%), 3.15 to 4mm (48-62%),4 to
5mm (5-8%) and D 50 of 3.25mm ± 0.25. The objective of this study is to ensure this
physicochemical quality of Moroccan TSP production.
During this study, we were able to identify and modify a number of parameters in
different production phases to improve the quality of TSP according to AFCOME, knowing
that each phase of the process is a client of the previous phase and supplier of the next one.
REFERENCES
[1] Malakooti, M., Sustainable agriculture and yield increment by optimum fertilizer
utilization in Iran, Agricultural Extension Publications, Iran, 2000.
[2] Arpana, N., et al., Journal of Applied Biology, 12(1-2), 23, 2002.
[3] Mehrvarz, S., et al., Agric. & Environ. Sci., 3(6), 855, 2008.
[4] Maene, L., Efficient fertilizer use and its role in increasing food production and
protecting the environment, The 6th AFA International Annual Conference, 2000.
[5] Altieri, M.A., Agroecology: the science of sustainable agriculture, Intermediate
Technology Publications Ltd (ITP), 1995.
[6] Chaouqi, N., et al., International Journal of Geomate, 12(34), 76, 2017.
[7] Compaoré, E., et al., Cahiers Agricultures, 10(2), 81, 2001.
[8] Tremblay, G., et al., Canadian Journal of Soil Science, 91(4), 637, 2011.
[9] Sencovici, M., Pehoiu, G., Ecology, Economics, Education and Legislation
Conference Proceedings, International Multidisciplinary Scientific GeoConference-
SGEM, I, 277, 2016.
[10] Pehoiu, G., Frinculeasa, M.N., Economics, Education and Legislation Conference
Proceedings, International Multidisciplinary Scientific GeoConference-SGEM, I, 735,
2015.
[11] Ispas, S., et al., Economics, Education and Legislation Conference Proceedings,
International Multidisciplinary Scientific GeoConference-SGEM, II, 1055, 2011.
[12] Murarescu, O., et al., Political Sciences, Law, Finance, Economics and Tourism,
International Multidisciplinary Scientific Conferences on Social Sciences and Arts,
IV, 59, 2014.
[13] Mazoyer, M., Roudart, L., Histoire des agricultures du monde, Du néolithique à la
crise contemporaine, Le Seuil, 2017.
www.josa.ro Chemistry Section
You might also like
- CATION AND ANION DETECTIONDocument6 pagesCATION AND ANION DETECTIONAshley Kim100% (1)
- Sci 207 Week 5 Assignment Final Lab ReportDocument6 pagesSci 207 Week 5 Assignment Final Lab ReportRoshondaGatesNo ratings yet
- Vortex-Breaking PDFDocument7 pagesVortex-Breaking PDFZeroRecoNo ratings yet
- Astm E3-11Document12 pagesAstm E3-11Alejandro OrtizNo ratings yet
- MDMW Apatite Rockphosphate04Document4 pagesMDMW Apatite Rockphosphate04licservernoidaNo ratings yet
- Tan 2021 IOP Conf. Ser. Earth Environ. Sci. 772 012047Document8 pagesTan 2021 IOP Conf. Ser. Earth Environ. Sci. 772 012047Ingrid ContrerasNo ratings yet
- Phosphate Recovery From Wastewater of Fertiliser Industries by Using Gypsum WasteDocument6 pagesPhosphate Recovery From Wastewater of Fertiliser Industries by Using Gypsum WasteJohnNo ratings yet
- SSP Modelling Paper PDFDocument5 pagesSSP Modelling Paper PDFJai GaneshNo ratings yet
- Sintesis Pati Sagu Ikatan Silang Fosfat Berderajat Substitusi Fosfat Tinggi Dalam Suasana AsamDocument7 pagesSintesis Pati Sagu Ikatan Silang Fosfat Berderajat Substitusi Fosfat Tinggi Dalam Suasana AsamNatassya LeoNo ratings yet
- 41132-Article Text-94450-1-10-20151019 PDFDocument9 pages41132-Article Text-94450-1-10-20151019 PDFvelisia22No ratings yet
- Environmental Impact and Management of Phosphogypsum (Review)Document38 pagesEnvironmental Impact and Management of Phosphogypsum (Review)Jesus Abelardo Figueira ViñaNo ratings yet
- Removal of The Ammonia Load of Landfill Leachate by Struvite Precipitation Using Low-Cost ReagentsDocument8 pagesRemoval of The Ammonia Load of Landfill Leachate by Struvite Precipitation Using Low-Cost ReagentsMario WhoeverNo ratings yet
- Recovery PhosphateDocument4 pagesRecovery PhosphateYuni HapsariNo ratings yet
- Almeida 2020Document9 pagesAlmeida 2020Nautam ParasanaNo ratings yet
- Determinare FosforDocument10 pagesDeterminare FosforDaniela CatanaNo ratings yet
- 1 s2.0 S1383586622026053 MainDocument10 pages1 s2.0 S1383586622026053 MainIkram ADNANENo ratings yet
- Removal of Phosphate Species From Solution by Adsorption Onto Calcite Used As Natural AdsorbentDocument6 pagesRemoval of Phosphate Species From Solution by Adsorption Onto Calcite Used As Natural AdsorbentArif HidayatNo ratings yet
- JurnalDocument21 pagesJurnalIndah Suci RamadhaniNo ratings yet
- GH - Tirpe, Olivia Paula TirpeDocument4 pagesGH - Tirpe, Olivia Paula TirpeLiv CBNo ratings yet
- Phosphorus SolubilityDocument8 pagesPhosphorus SolubilityQuality LabNo ratings yet
- Removal of Phosphate From Wastewater Using Low-Cost AdsorbentsDocument7 pagesRemoval of Phosphate From Wastewater Using Low-Cost AdsorbentsInternational Journal of Engineering Inventions (IJEI)No ratings yet
- The Use of Phosphorus and Other Soil Amendments FoDocument10 pagesThe Use of Phosphorus and Other Soil Amendments FoLaura GonzálezNo ratings yet
- Remediation of Salt-Affected Soil by The Addition of Organic Matter - An Investigation Into Improving Glutinous Rice ProductivityDocument5 pagesRemediation of Salt-Affected Soil by The Addition of Organic Matter - An Investigation Into Improving Glutinous Rice ProductivityRichar Manuel Simanca FontalvoNo ratings yet
- Absorcion AtomicaDocument10 pagesAbsorcion AtomicaCelestino Celestino MontalvoNo ratings yet
- Crystal Habit Modifiers For Enhanced Filtration of PhosphogypsumDocument14 pagesCrystal Habit Modifiers For Enhanced Filtration of PhosphogypsummahaNo ratings yet
- Production of Polyhydroxyalkanoates From Sludge Palm Oil Using Pseudomonas Putida S12Document5 pagesProduction of Polyhydroxyalkanoates From Sludge Palm Oil Using Pseudomonas Putida S12Shandyka YudhaNo ratings yet
- Investigation On The Phosphoric Acid Production From Low Grade Phosphorites With High Content of MagnesiumDocument6 pagesInvestigation On The Phosphoric Acid Production From Low Grade Phosphorites With High Content of MagnesiumamirNo ratings yet
- Belum Di ReviewDocument7 pagesBelum Di ReviewTatang KelanaNo ratings yet
- Manuscript Final 28-7-15 PlaintextDocument24 pagesManuscript Final 28-7-15 PlaintextPrithviNo ratings yet
- Phosphorus Recovery and Leaching of Trace Elements From Incinerated Sewage Sludge Ash (ISSA)Document33 pagesPhosphorus Recovery and Leaching of Trace Elements From Incinerated Sewage Sludge Ash (ISSA)JohnNo ratings yet
- 178 JMES 2279 BenhsinatDocument9 pages178 JMES 2279 BenhsinatGiussepi Ali Jhonatan Mamani PacoNo ratings yet
- Combined Procedure of Metal Removal and Recovery of T - 2021 - Journal of GeocheDocument9 pagesCombined Procedure of Metal Removal and Recovery of T - 2021 - Journal of GeocheEnzo GonzalezNo ratings yet
- Regenerative Role of The Red Phosphorus in The Couple HI/P'Document6 pagesRegenerative Role of The Red Phosphorus in The Couple HI/P'spNo ratings yet
- Photosynthesis and chlorophyll content of soybeans on acidic growth medium with aluminum saltsDocument8 pagesPhotosynthesis and chlorophyll content of soybeans on acidic growth medium with aluminum saltsPasih UlisanNo ratings yet
- Laju Fotosintesis Dan Kandungan Klorofil Kedelai Pada Media Tanam Masam Dengan Pemberian Garam AluminiumDocument8 pagesLaju Fotosintesis Dan Kandungan Klorofil Kedelai Pada Media Tanam Masam Dengan Pemberian Garam AluminiumDzul AdamNo ratings yet
- Experimental Study of The Removal of Copper From Aqueous Solutions by Adsorption Using SawdustDocument8 pagesExperimental Study of The Removal of Copper From Aqueous Solutions by Adsorption Using SawdustchikubadgujarNo ratings yet
- Gravimetric Determination of Phosphorus in Fertilizer SamplesDocument5 pagesGravimetric Determination of Phosphorus in Fertilizer SamplesCatherine PascuaNo ratings yet
- Low-pH Cement Design for Nuclear Waste StorageDocument15 pagesLow-pH Cement Design for Nuclear Waste StorageMss BranchesNo ratings yet
- FERTILIZER ELEMENT ANALYSISDocument2 pagesFERTILIZER ELEMENT ANALYSIShafiz riasatNo ratings yet
- Simultaneous Biohythane Production and Sulfate Remova 2020 International JouDocument12 pagesSimultaneous Biohythane Production and Sulfate Remova 2020 International JouNurul Fatin UmairahNo ratings yet
- Phosphate Solubilization Characteristics of Efficient Nitrogen Fixing SoilDocument11 pagesPhosphate Solubilization Characteristics of Efficient Nitrogen Fixing SoilJoha MamarandiNo ratings yet
- Synthesis and Evaluation Catalytic Efficiency of Perovskite-Type Oxide Nanopowders in Removal of Bromocresol Purple From Aqueous SolutionDocument12 pagesSynthesis and Evaluation Catalytic Efficiency of Perovskite-Type Oxide Nanopowders in Removal of Bromocresol Purple From Aqueous SolutionAmin MojiriNo ratings yet
- Optimization of Nitric Acid Leaching of Rare Earth Elements FromDocument11 pagesOptimization of Nitric Acid Leaching of Rare Earth Elements FromIslom MurodovNo ratings yet
- Langmuir, Freundlich and BET Adsorption Isotherm Studies For Zinc Ions Onto Coal Fly AshDocument8 pagesLangmuir, Freundlich and BET Adsorption Isotherm Studies For Zinc Ions Onto Coal Fly AshInternational Journal of Application or Innovation in Engineering & ManagementNo ratings yet
- Paper 19Document6 pagesPaper 19Editor IJSETNo ratings yet
- Introduction-WPS OfficeDocument5 pagesIntroduction-WPS OfficeHIMANSHU VARDHANNo ratings yet
- Characterization and Purification of Waste Phosphogypsum To Make It Suitable For Use in The Plaster and The Cement IndustryDocument12 pagesCharacterization and Purification of Waste Phosphogypsum To Make It Suitable For Use in The Plaster and The Cement Industryritesh malaniNo ratings yet
- ApatitDocument9 pagesApatittrinh xuan hiepNo ratings yet
- Phosphorus Industry Processes and ProductsDocument38 pagesPhosphorus Industry Processes and ProductsS S S REDDY100% (1)
- The Status of Phosphate (PO) Anion Concentration in Waste Water From Six Selected Undetermined Areas of Guyana Using A Spectrophotometric MethodDocument11 pagesThe Status of Phosphate (PO) Anion Concentration in Waste Water From Six Selected Undetermined Areas of Guyana Using A Spectrophotometric MethodidaayudwitasariNo ratings yet
- Processes 11 01753Document25 pagesProcesses 11 01753JanainaNo ratings yet
- Nitrogen and Phosphorus Recovery From Wastewater: Water Pollution (S Sengupta, Section Editor)Document12 pagesNitrogen and Phosphorus Recovery From Wastewater: Water Pollution (S Sengupta, Section Editor)JohnNo ratings yet
- Environmental Tech Journal Article on Carbon/FexOy Composites for Wastewater TreatmentDocument42 pagesEnvironmental Tech Journal Article on Carbon/FexOy Composites for Wastewater TreatmentkenedymcNo ratings yet
- RDR 3Document2 pagesRDR 3Andre Jet Russo RuizNo ratings yet
- PhotodegradationDocument8 pagesPhotodegradationLuminita AndronicNo ratings yet
- Prediction of Calcium Phosphate Generation and BehDocument15 pagesPrediction of Calcium Phosphate Generation and BehAdib Hossain SijanNo ratings yet
- Naoh Modification of Persimmon Powder-Formaldehyde Resin To Enhance Cu and PB Removal From Aqueous SolutionDocument10 pagesNaoh Modification of Persimmon Powder-Formaldehyde Resin To Enhance Cu and PB Removal From Aqueous SolutionSoussou PerlaNo ratings yet
- Phenol 3 PDFDocument10 pagesPhenol 3 PDFsegNo ratings yet
- Saindrenan 1985Document7 pagesSaindrenan 1985cesarNo ratings yet
- Haiming 1-2014Document17 pagesHaiming 1-2014Febri Daris Faidatur RohmahNo ratings yet
- Screening For Phosphate Solubilizing Bacteria and Optimum of Bacterial Cultivation by Response Surface MethodologyDocument14 pagesScreening For Phosphate Solubilizing Bacteria and Optimum of Bacterial Cultivation by Response Surface Methodologymuler_biotech8366No ratings yet
- Perfluorinated Chemicals (PFCs): Contaminants of ConcernFrom EverandPerfluorinated Chemicals (PFCs): Contaminants of ConcernNo ratings yet
- Proper Piping For Vacuum SystemsDocument5 pagesProper Piping For Vacuum SystemsDowni Oader100% (1)
- Fertilizer Industry Handbook - October 2018 (Slides Only)Document97 pagesFertilizer Industry Handbook - October 2018 (Slides Only)ASDFG21100% (1)
- SamplingDocument16 pagesSamplingbradburywillsNo ratings yet
- Sodium Hypo FRP SpecDocument6 pagesSodium Hypo FRP SpecZeroRecoNo ratings yet
- Booklet 5 Final PDFDocument44 pagesBooklet 5 Final PDFMilan BanerjeeNo ratings yet
- Air Side Pressure Drop in Plate Finned Tube Heat Exchangers PDFDocument14 pagesAir Side Pressure Drop in Plate Finned Tube Heat Exchangers PDFZeroRecoNo ratings yet
- Scilab BeginnersDocument33 pagesScilab BeginnersCarlos Soza RossNo ratings yet
- Norsok ST 2001Document131 pagesNorsok ST 2001mamounsdNo ratings yet
- Yara Fertilizer Industry Handbook: February 2014Document90 pagesYara Fertilizer Industry Handbook: February 2014douglasminasNo ratings yet
- Water97 v13Document8 pagesWater97 v13techkasambaNo ratings yet
- 16.050 Thermal Energy: Page 1 of 6Document6 pages16.050 Thermal Energy: Page 1 of 6ZeroRecoNo ratings yet
- Air Side Pressure Drop in Plate Finned Tube Heat Exchangers PDFDocument14 pagesAir Side Pressure Drop in Plate Finned Tube Heat Exchangers PDFZeroRecoNo ratings yet
- The Critical Height of A Liquid Being Drained From The TankDocument5 pagesThe Critical Height of A Liquid Being Drained From The TankZeroRecoNo ratings yet
- 16.050 Thermal Energy: Page 1 of 2Document2 pages16.050 Thermal Energy: Page 1 of 2ZeroRecoNo ratings yet
- Aerospace Science and Technology: Dheeraj Agarwal, Prateep Basu, T. John Tharakan, A. SalihDocument6 pagesAerospace Science and Technology: Dheeraj Agarwal, Prateep Basu, T. John Tharakan, A. SalihNicolas PironnetNo ratings yet
- Air Entrainment in Vertical DropshaftsDocument199 pagesAir Entrainment in Vertical DropshaftsZeroRecoNo ratings yet
- Tables - TechnicalProperties - EN 10088 PDFDocument24 pagesTables - TechnicalProperties - EN 10088 PDFeugenio.gutenbertNo ratings yet
- Configure VS Code For Microsoft CDocument12 pagesConfigure VS Code For Microsoft CZeroRecoNo ratings yet
- Epa Tank Resuspension PDFDocument101 pagesEpa Tank Resuspension PDFZeroRecoNo ratings yet
- Epa Tank Resuspension PDFDocument101 pagesEpa Tank Resuspension PDFZeroRecoNo ratings yet
- Emissivity TableDocument5 pagesEmissivity TableZeroRecoNo ratings yet
- AV041R0 ACS Types of Rotors Product SheetDocument2 pagesAV041R0 ACS Types of Rotors Product SheetZeroRecoNo ratings yet
- Solubility of CO2 in WaterDocument26 pagesSolubility of CO2 in WaterZeroRecoNo ratings yet
- CG 160729 What Does Good Control Room Design Look LikeDocument10 pagesCG 160729 What Does Good Control Room Design Look LikeRavi JoshiNo ratings yet
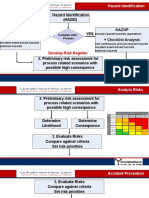
- Hazard Identification (Hazid) NO YES: Checklist Analysis Hazop + Checklist AnalysisDocument7 pagesHazard Identification (Hazid) NO YES: Checklist Analysis Hazop + Checklist AnalysisZeroRecoNo ratings yet
- Water97 v13Document8 pagesWater97 v13techkasambaNo ratings yet
- 1108 PDFDocument4 pages1108 PDFzacuragyNo ratings yet
- Facility Layout PlanningDocument5 pagesFacility Layout PlanningRahul GargNo ratings yet
- Data Faktur Apotik Mutiara InsaniDocument13 pagesData Faktur Apotik Mutiara Insaniapotek mutiarainsaniNo ratings yet
- 4.2 Procesos de Pulpeo - Pulpeo QuímicoDocument38 pages4.2 Procesos de Pulpeo - Pulpeo QuímicoMiguel MontielNo ratings yet
- Cost of Corrosion: Key Performance IndicatorDocument4 pagesCost of Corrosion: Key Performance IndicatorJuan Carlos Contreras CherresNo ratings yet
- Alkaloid & Its General PropertiesDocument4 pagesAlkaloid & Its General Propertiesshankul kumar100% (9)
- Set 11 HaloalkanesDocument2 pagesSet 11 HaloalkanesNurul FarhanaNo ratings yet
- Student Exploration of Chemical EquationsDocument7 pagesStudent Exploration of Chemical EquationsDanitza RojasNo ratings yet
- Velosit CW 111Document4 pagesVelosit CW 111Miguel AragonNo ratings yet
- Aerospace Material Specification: Plating, Nickel General PurposeDocument8 pagesAerospace Material Specification: Plating, Nickel General PurposeSURYAS63No ratings yet
- C2 - ICON OT - 5 (Mains Model)Document8 pagesC2 - ICON OT - 5 (Mains Model)NIVEDITA CHAKRABORTYNo ratings yet
- Department of Chemistry Faculty of Science and Mathematics: SEMESTER 1 SESSION 2021/2022Document4 pagesDepartment of Chemistry Faculty of Science and Mathematics: SEMESTER 1 SESSION 2021/2022Jojie KimaraNo ratings yet
- Kondawar 2017 Solvent Free Glycerol TransesterifiDocument11 pagesKondawar 2017 Solvent Free Glycerol TransesterifiElisabeta StamateNo ratings yet
- Science7 ST Q1Document3 pagesScience7 ST Q1EILEEN JOY NOCEDANo ratings yet
- Performance Data CurveDocument57 pagesPerformance Data Curveedwin nolberto100% (1)
- Blood Cell Counter GuideDocument5 pagesBlood Cell Counter GuideAmirNo ratings yet
- Biochemical MeasurementsDocument25 pagesBiochemical Measurementsulaga nathanNo ratings yet
- Guidelines for Packaging, Labelling and Storage of Scheduled WastesDocument35 pagesGuidelines for Packaging, Labelling and Storage of Scheduled WastesShashaNo ratings yet
- An Experimental Study On Operating Conditions of 2 Ethylhexanol Operating ProcessDocument30 pagesAn Experimental Study On Operating Conditions of 2 Ethylhexanol Operating ProcessShay BlueNo ratings yet
- Group 3 CementDocument16 pagesGroup 3 Cementshuckss taloNo ratings yet
- Virtual Lab. Calculating Molarity Part 1Document2 pagesVirtual Lab. Calculating Molarity Part 1DomNo ratings yet
- Experiment 1 1Document57 pagesExperiment 1 1Christine Mae VeaNo ratings yet
- Yohimbine InjectionDocument1 pageYohimbine InjectionKasidit SornchaiNo ratings yet
- Organic ChemistryDocument2,476 pagesOrganic Chemistrytilakmirle75% (4)
- Mark Scheme (Results) January 2022Document25 pagesMark Scheme (Results) January 2022윤소리No ratings yet
- Iso 8502-6-2020Document18 pagesIso 8502-6-2020pedro davilaNo ratings yet
- European Patent SpecificationDocument13 pagesEuropean Patent SpecificationWSERNo ratings yet
- Improve Landing Gear Corrosion ProtectionDocument26 pagesImprove Landing Gear Corrosion ProtectionImpress Production PlanningNo ratings yet
- Transcriptomic Analysis of The Under Oxidative Stress: Levilactobacillus Brevis 47f StrainDocument9 pagesTranscriptomic Analysis of The Under Oxidative Stress: Levilactobacillus Brevis 47f StrainMohammed SherifNo ratings yet