Professional Documents
Culture Documents
Solid State Chemistry-4: Dr. Shaista Ali CHEM-3207
Uploaded by
Muhammad Shehzad Haider0 ratings0% found this document useful (0 votes)
6 views45 pagesoptical properties of solid solutions
Original Title
10-optical properties
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentoptical properties of solid solutions
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views45 pagesSolid State Chemistry-4: Dr. Shaista Ali CHEM-3207
Uploaded by
Muhammad Shehzad Haideroptical properties of solid solutions
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 45
Solid State Chemistry-4
Dr. Shaista Ali
CHEM-3207
OPTICAL
PROPERTIES
Wave Theory of Light: Huygens, Fresnel, Hertz, Maxwell
The light field
consists of a an
electric and a
magnetic field
component
But: The light field can take up or
transfer energy only in
packages (light quantums =
photons)
“Wave-particle-dualism”
Proof for above hypothesis:
• Photons liberate electrons from an electrode, if their frequency
> E/h (critical frequency)
• Derivation of Planck„s radiation law for cavity radiation is based on the
quantization of
the energy of the light field (Planck, 1900)
Electromagnetic radiation consists of
wave packages (photons) with
discrete energy E and
momentum p
INTERACTION OF
LIGHT WITH
ATOMS
Interaction of light with Atoms
Atom absorbs photon of light undergoes
transition to higher energy level using
selection rules.
3s of Na-atom can absorb and go to 3p
level not 3d or 4s, indicates the concept of
– Allowed transitions
– Forbidden transitions
Absorption
Spontaneous emission
Induced or simulated emission
Coherent- photon emitted are in same direction as
photon inducing emission resulting in beam of light
Non-radiative Transitions: the atom may
collide with another atom, losing energy in the process or
giving energy to its surroundings in the form of
vibrational energy.
In a crystal selection rules are modified:
electron outside a closed shell (Ti3+)
In the free ion, the five 3d orbitals all have the same
energy.
In a crystal, these levels are split; for example, if the
ion occupied an octahedral hole, the 3d levels would be
split into a lower, triply degenerate (t2g) level and a
higher, doubly degenerate (eg) level.
RUBY LASER
Ruby Laser
Ruby is Corundum (form of
Al2O3) with 0.05-0.5% Cr+3-ions.
The three 3d electrons of Cr+3
will absorb energy and move to
higher energy level.
In Ruby-Forbidden Transition
(doubly forbidden)
Cr+3-ions absorb light and go to
state 3 and 4, then undergo
transition to state 2 and then to
ground state , resulting photons
will travel through ruby.
The reflected photons induce further
emission and by this means, an appreciable
beam of coherent light is built up.
PHOSPHORS IN
FLUORESCENT
LIGHTS
Phosphors are solids that absorb energy and re-
emit it as light.
Impurity in host lattice.
Application: 1) Plasma in TV
2) Fluorescent Light blubs
Fluorescent lights- produce radiation in the ultraviolet (254
nm) by passing an electric discharge through a low pressure of
mercury vapour. The tube is coated inside with a white powder that
absorbs the ultraviolet light and emits visible radiation.
Most common fluorescent alkaline earth
halophosphates, such as 3Ca3(PO4)2.CaF2.
In laser- usual dopant are transition metal or
lanthanide ions
The incident light is of lower energy than
the emitted light and the process is known
as upconversion.
An ion absorbs a photon of the incident
radiation and goes to an excited state.
It then transfers most of the energy either
to another state of that ion or to the excited
state of another ion.
If this second excited state is metastable, it
has time to absorb another photon before it
spontaneously emits radiation and returns
to a lower state.
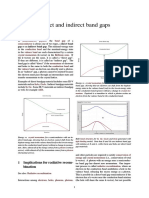
DIRECT/INDIRECT
BAND GAPS
If in some solids the level at the top of the
valence band and that at the bottom of the
conduction band have the same wave
vector. There is then an allowed transition
at the band gap energy. Such solids are
said to have a direct band gap.
If the direct transition from the top of the
valence band to the bottom of the
conduction band is forbidden. These solids
are said to have an indirect band gap.
Many metals have strong
transitions between the
conduction band and a
higher energy band, which
lead to their characteristic
metallic sheen.
Gold and copper have
strong absorption bands due
to the excitation of d band
electrons to the s/p
conduction band. In these
elements, the d band is full
and lies some way below
the Fermi level.
LEDs
Quantum Wells: Blue Lasers
The active region of GaN lasers consists of GaN
containing several thin layers (3–4 nm thick) of
indium-doped GaN, InxGa1–xN. The addition of
indium reduces the band gap within the thin
layers, so that the bottom of the conduction band
is at a lower energy than that of the bulk GaN.
The electrons in this conduction band are
effectively trapped because they need to gain
energy from an external source to pass into the
conduction band of the bulk GaN.
The trapped electrons behave like particles in a
box and such boxes are quantum wells.
The active region containing the quantum
wells is sandwiched between layers of n-
doped and p-doped GaN and aluminium-
doped GaN, AlyGa1–yN, which provide the
electrons entering the quantum well and
keep them confined to the active region.
Blu-ray disks, introduced in 2006, are
DVD-like devices using blue lasers to read
the disk.
REFRACTION
Passage of light through medium
It depends on refractive index of medium,
as per Snell’s law radiation bend in 2
medium is
n1sin θ1 = n2sin θ2,
In a molecule, the amount of response from
the electrons depends on how tightly bound
the electrons are to the nucleus.
This property is called the polarisability and
is higher for large ions with low charge, for
example, Cs+ and I−, than for small, highly
charged ions such as Al3+.
Calcite
Calcite is stable
polymorph CaCO3.
It is birefringence
means having different
polarisabilities in the
directions of different
crystal axes and hence
different refractive
indices for light
polarised perpendicular
to these axes.
OPTICAL FIBRES
Optical fibres are used to transmit light in the way that
metal wires are used to transmit electricity, e.g. telephone
call.
The intensity, the time between pulses and the length of
the pulse can be modified to convey the contents of the
call in coded form.
The first requirement is that the laser beam keeps within
the fibre.
The refractive index can be varied by adding selected
impurities.
The imperfections cause scattering of the light of a type
known as Rayleigh scattering (1/λ4).
The third source of energy loss is absorption of light by
the fibre.
PHOTONIC
CRYSTALS
PHOTONIC CRYSTALS
Photonic crystals have been hailed as the
optical equivalent of semiconductors.
A photonic crystal consists of a periodic
arrangement of two materials of different
refractive indexes.
At each boundary between the two
materials, light, or other electromagnetic
radiation, will refract and partly reflect.
Photonic band gap.
METAMATERIALS:
CLOAKS OF INVISIBILITY
You might also like
- Band Theory of SolidsDocument26 pagesBand Theory of SolidsDizney Lobaton EsparteroNo ratings yet
- RADIOGRAPHY PRINCIPLESDocument63 pagesRADIOGRAPHY PRINCIPLESaasattiNo ratings yet
- Direct and Indirect Band GapsDocument5 pagesDirect and Indirect Band GapsSenthil Siva SubramanianNo ratings yet
- (Asce) SC 1943-5576 0000367 PDFDocument5 pages(Asce) SC 1943-5576 0000367 PDFirmakNo ratings yet
- L2 Che101Document16 pagesL2 Che101Musa Ahammed MahinNo ratings yet
- Optical Properties of Materials: Reflection, Refraction, Absorption & MoreDocument18 pagesOptical Properties of Materials: Reflection, Refraction, Absorption & MoreAshish Manatosh BarikNo ratings yet
- FB-MultiPier Vs LPileDocument33 pagesFB-MultiPier Vs LPileabhinavka123No ratings yet
- Uv Visible SpectrosDocument14 pagesUv Visible SpectrosDevanshi JadaunNo ratings yet
- Optical Properties & CharacteristicsDocument21 pagesOptical Properties & CharacteristicsRogelyn JosolNo ratings yet
- Chapter 8: Momentum, Impulse, and Collisions ExplainedDocument18 pagesChapter 8: Momentum, Impulse, and Collisions ExplainedJulius CodillaNo ratings yet
- Laser TherapyDocument8 pagesLaser Therapyinrmpt77No ratings yet
- Laser TherapyDocument8 pagesLaser Therapytagewub4No ratings yet
- Lecture 07 - OM - EM PDFDocument33 pagesLecture 07 - OM - EM PDFHemant KumarNo ratings yet
- Lasers: Characteristics of A LaserDocument9 pagesLasers: Characteristics of A Lasersreekrish108No ratings yet
- Physical Elec NotesDocument22 pagesPhysical Elec NotesSimion OngoriNo ratings yet
- Lecture 7 & 8: Bonding and band structure in materialsDocument35 pagesLecture 7 & 8: Bonding and band structure in materialsPunit Yadav YadavNo ratings yet
- Electron Configurations- Ionization EnergiesDocument33 pagesElectron Configurations- Ionization EnergiesRayan BotanyNo ratings yet
- Semiconductors Create and Detect Light for OptoelectronicsDocument5 pagesSemiconductors Create and Detect Light for OptoelectronicsJoseGarciaRuizNo ratings yet
- Unit 2 The Electronic Structure of Atoms and The Periodic TableDocument22 pagesUnit 2 The Electronic Structure of Atoms and The Periodic TableAragorn ChanNo ratings yet
- Uv Visible SpectrosDocument14 pagesUv Visible SpectrosDevanshi JadaunNo ratings yet
- Structure of Atom Class 11Document74 pagesStructure of Atom Class 11Komal VermaNo ratings yet
- P - 3.12 - RM Kind of LasersDocument8 pagesP - 3.12 - RM Kind of Lasersdeekshithasweety0No ratings yet
- Direct and Indirect Band GapsDocument4 pagesDirect and Indirect Band GapsSai Swetha KVNo ratings yet
- L13 Optical CharacterizationDocument35 pagesL13 Optical CharacterizationVivek BelaNo ratings yet
- Optical Material and SensorsDocument28 pagesOptical Material and SensorsdinasharanaNo ratings yet
- Lec-PH301 18 Optical Sources-3 15.09.2023Document32 pagesLec-PH301 18 Optical Sources-3 15.09.2023Latu BharaliNo ratings yet
- Principle and Working of A Semiconductor LaserDocument17 pagesPrinciple and Working of A Semiconductor LaserMANISH KUMAR 20SCSE1290075No ratings yet
- Photovoltaic Systems GuideDocument98 pagesPhotovoltaic Systems GuideNoman Ahmed KhanNo ratings yet
- Absorptivity and Emissivity ExplainedDocument7 pagesAbsorptivity and Emissivity ExplainedAlex AntiaNo ratings yet
- EEE132 Electronic Devices: Prof Syed Idris Syed Hassan MR Arjuna Marzuki Mrs Norlaili Mohd NohDocument57 pagesEEE132 Electronic Devices: Prof Syed Idris Syed Hassan MR Arjuna Marzuki Mrs Norlaili Mohd NohJhiGz Llausas de GuzmanNo ratings yet
- Optical Properties SummaryDocument6 pagesOptical Properties Summaryrovic.esprelaNo ratings yet
- Department of Mathematics and Natural Sciences CHE 101: Introduction To Chemistry Dr. Zayed Bin Zakir ShawonDocument13 pagesDepartment of Mathematics and Natural Sciences CHE 101: Introduction To Chemistry Dr. Zayed Bin Zakir ShawonSYEDA UMME SALMANo ratings yet
- r19 Edc Notes - All UnitsDocument165 pagesr19 Edc Notes - All Unitsbaburao_kodavatiNo ratings yet
- Semiconductor Theory and Devices 11.1 - 11.2Document35 pagesSemiconductor Theory and Devices 11.1 - 11.2jamalur.lNo ratings yet
- Atomic Structure and The Periodic TableDocument11 pagesAtomic Structure and The Periodic TableShacquelle WilsonNo ratings yet
- EENG 292 Lec1Document6 pagesEENG 292 Lec1Simion OngoriNo ratings yet
- Electrical Transport in SolidsDocument130 pagesElectrical Transport in SolidsSaroshan DeshapriyaNo ratings yet
- Excess Carriers and Junction Concepts: Unit IIIDocument48 pagesExcess Carriers and Junction Concepts: Unit IIIThamizharasanNo ratings yet
- Electronic StructureDocument26 pagesElectronic StructureDavidson ChanNo ratings yet
- Physics Solid State NotesDocument9 pagesPhysics Solid State NotesMatheus SiqueiraNo ratings yet
- X-RaysDocument50 pagesX-RaysAqilNo ratings yet
- Various Tools and Techniques Used For Structural ElucidationDocument28 pagesVarious Tools and Techniques Used For Structural ElucidationAvinashNo ratings yet
- Metallic Bonding - 3 Types of SemiconductorsDocument17 pagesMetallic Bonding - 3 Types of Semiconductorssherin joyNo ratings yet
- LasersDocument4 pagesLasersOpenaiNo ratings yet
- UV-Visible Spectroscopy: Dr. V. KrishnakumarDocument32 pagesUV-Visible Spectroscopy: Dr. V. KrishnakumarDarpan KumarNo ratings yet
- S/Sciences/Physics/Optics/Lasertut Orial/Creating/Creating - HTMDocument13 pagesS/Sciences/Physics/Optics/Lasertut Orial/Creating/Creating - HTMSuvankar ChakrabortyNo ratings yet
- Laser Physics Course OverviewDocument22 pagesLaser Physics Course OverviewFAKIHA GULZAR BS PhysicsNo ratings yet
- Plasm On IcsDocument15 pagesPlasm On Icschouht98477714No ratings yet
- Superconductors to Lasers: A Guide to Radiation Sources and Particle InteractionsDocument25 pagesSuperconductors to Lasers: A Guide to Radiation Sources and Particle InteractionsMohamed ElashriNo ratings yet
- 546 5686798651313311232164897987lijdcoihsdiuhgfieygDocument24 pages546 5686798651313311232164897987lijdcoihsdiuhgfieygędën hãzárdNo ratings yet
- Optical PropertiesDocument27 pagesOptical PropertiespotterheadNo ratings yet
- Solar ElectricityDocument40 pagesSolar Electricitysonu kumarNo ratings yet
- Analysis of UV-Visible Spectroscopy TechniquesDocument32 pagesAnalysis of UV-Visible Spectroscopy TechniquesRafael HenriqueNo ratings yet
- A Seminar On Energy Bands and Gaps in Semiconductor: Mr. M. VenkaiahDocument23 pagesA Seminar On Energy Bands and Gaps in Semiconductor: Mr. M. VenkaiahA S M Younus Bhuiyan SabbirNo ratings yet
- Chapter No 5 Work SheetDocument10 pagesChapter No 5 Work SheetNabeel RazaNo ratings yet
- Bohr Model Explains Atomic Emission SpectraDocument45 pagesBohr Model Explains Atomic Emission Spectradineshk3No ratings yet
- ChemDocument24 pagesChemDaniel ConcessaoNo ratings yet
- 4.1 Basics and Kirchoff's LawsDocument38 pages4.1 Basics and Kirchoff's LawsdkbradleyNo ratings yet
- Lecture 2 - CHEM F111 - 1sem 2019-2020 - Quantum ChemDocument32 pagesLecture 2 - CHEM F111 - 1sem 2019-2020 - Quantum ChemShiva HarshithNo ratings yet
- RT BasicsDocument27 pagesRT BasicsAwais JamilNo ratings yet
- Maximum: LightingDocument1 pageMaximum: Lightingreacharunk100% (1)
- Chapter 9Document57 pagesChapter 9Pavan PonnadaNo ratings yet
- 1-2semi Cond, Defects ppt1Document22 pages1-2semi Cond, Defects ppt1Muhammad Shehzad HaiderNo ratings yet
- The Crystalline Solid State: Monday, October 19, 2015Document19 pagesThe Crystalline Solid State: Monday, October 19, 2015Muhammad Shehzad HaiderNo ratings yet
- Inorganic Metal Oxides and Reduction of Environmental Pollution Using NanoparticlesDocument29 pagesInorganic Metal Oxides and Reduction of Environmental Pollution Using NanoparticlesMuhammad Shehzad HaiderNo ratings yet
- Inorganic metal oxides and reduction of environmental pollution using nanoparticlesDocument32 pagesInorganic metal oxides and reduction of environmental pollution using nanoparticlesMuhammad Shehzad HaiderNo ratings yet
- Exploring Quantum Angular MomentumDocument4 pagesExploring Quantum Angular MomentumLeon DobrzinskyNo ratings yet
- 1st Year Physics All in One Notes-1-87Document87 pages1st Year Physics All in One Notes-1-87Zoya NaeemNo ratings yet
- Electrical Conductivity and Dielectric Behaviour of Nanocrystalline NiFe2Document18 pagesElectrical Conductivity and Dielectric Behaviour of Nanocrystalline NiFe2चन्द्रभाल सिंहNo ratings yet
- Electromagnetic Theory Basic PDFDocument29 pagesElectromagnetic Theory Basic PDFPranay ShuklaNo ratings yet
- Physical and Transport Properties For Simulation (CC-FLASH) Guide ChemCad Manual55Document108 pagesPhysical and Transport Properties For Simulation (CC-FLASH) Guide ChemCad Manual55agnotts09No ratings yet
- Tribological Behavior of Silicon Nitride MaterialsDocument10 pagesTribological Behavior of Silicon Nitride Materialsrahil7860No ratings yet
- Tool of The Complete Optimal Control For Variable Speed Electrical DrivesDocument36 pagesTool of The Complete Optimal Control For Variable Speed Electrical DrivesrijilpoothadiNo ratings yet
- STPM 2013 Sem 1Document7 pagesSTPM 2013 Sem 1nurulNo ratings yet
- Half Wave DipoleDocument3 pagesHalf Wave Dipolejoydeep1233% (3)
- Catastrophic Failure of Large Storage TanksDocument12 pagesCatastrophic Failure of Large Storage Tankszepol051No ratings yet
- Clear Identification of Nakamura (Nakamura, 2000) PDFDocument9 pagesClear Identification of Nakamura (Nakamura, 2000) PDFEvaNo ratings yet
- UV Spectroscopy: Principles and ApplicationsDocument20 pagesUV Spectroscopy: Principles and ApplicationsAMOGH DAHITULENo ratings yet
- Forms of EnergyDocument10 pagesForms of EnergyTrollzzz dogeNo ratings yet
- Pap 042Document12 pagesPap 042Elham MashhudiNo ratings yet
- First Order Differential Equations in Chemical Engineering ProblemsDocument3 pagesFirst Order Differential Equations in Chemical Engineering ProblemsAlexis JaraNo ratings yet
- Atomistics Course FileDocument34 pagesAtomistics Course FileVee KayNo ratings yet
- AQA Physics Topic 4 Atomic Structure Knowledge OrganiserDocument2 pagesAQA Physics Topic 4 Atomic Structure Knowledge Organisergundavannessa27No ratings yet
- Design 1 HeatExchangerDocument6 pagesDesign 1 HeatExchangerBooth DeschanelNo ratings yet
- Fundamental Physics NotesDocument5 pagesFundamental Physics NotesSNEHA TIMSINANo ratings yet
- 21 MagnetismDocument5 pages21 MagnetismYani AhmadNo ratings yet
- 1st Year Formula SheetDocument14 pages1st Year Formula SheetSaim SultanNo ratings yet
- Phy Cet Wei 23Document4 pagesPhy Cet Wei 23endtimes066xNo ratings yet
- L29 - Vapor Compression RefrigerationDocument14 pagesL29 - Vapor Compression RefrigerationAnonymous 5HYsyrddpNo ratings yet
- Pumps for High GOR and Viscous FluidsDocument20 pagesPumps for High GOR and Viscous FluidssaeedNo ratings yet
- Bio Chemical Separation Assignment No. 4Document3 pagesBio Chemical Separation Assignment No. 4Nikhil TanwarNo ratings yet
- Thesis Dynamic Stiffness Formulations For CompositesDocument172 pagesThesis Dynamic Stiffness Formulations For Compositesajaythegem7No ratings yet
- Analysis of StructuresDocument30 pagesAnalysis of Structurescontrax8No ratings yet