Professional Documents
Culture Documents
Selected Answers For Exercises: Product KG Waste KG E
Uploaded by
keatyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Selected Answers For Exercises: Product KG Waste KG E
Uploaded by
keatyCopyright:
Available Formats
Selected answers for exercises 1 www.catalysisbook.
org
This file contains answers to selected questions from the book Catalysis: Concepts and Green
Applications, ISBN 978-3-527-31824-7. Additional teaching material is available free of
charge at www.catalysisbook.org
There has been quite a lot of feedback regarding the exercises and answers. Basically,
colleagues express mixed feelings on the issue of placing the answers to exercises on the
internet. After some discussion, I have decided to include answers to selected questions only.
In some cases, I included also references to background material and further reading, where
possible with links to free-access websites
Selected answers for Chapter 1
A1.1 Green chemistry principles relating to the Atom Economy and the E-factor:
• I Prevent waste instead of treating it.
• II Design atom-efficient synthetic methods.
• V Minimise the use of auxiliary reagents and solvents.
• VIII Avoid unnecessary derivatisation.
• IX Replace stoichiometric reagents with catalytic cycles.
Principles relating to the environmental quotient, Q:
• III Choose synthetic routes using non-toxic compounds where possible.
• IV Design new products that preserve functionality while reducing toxicity.
• VII Preferably use renewable raw materials.
• IX Replace stoichiometric reagents with catalytic cycles.
• X Design new products with biodegradable capabilities.
• XII Choose feedstocks and design processes that minimise the chance of accidents.
A1.2 Example 1:
2C6H5I + Cu → C6H5–C6H5 + CuI2 (100% yield)
Here no reactant is left over, since the conversion is 100%. Thus, for every mol of biphenyl
product, we obtain one mol of CuI2 waste. First, we calculate the number of moles in one kg
of product:
Mwbiphenyl, or C12H10, = 154 gr/mol, so 1 kg biphenyl = 6.5 mol biphenyl. This means that
every kg of biphenyl product will give us 6.5 mol of CuI2, which is equivalent to 6.5 × 317.5 =
2063.75 gr CuI2.
kg ( waste) 2.064
The overall E-factor is therefore: E factor = = = 2.064
kg ( product ) 1.000
Example 2:
2C6H5Br + Cu → C6H5–C6H5 + CuBr2 (85% yield)
Importantly, we see that this reaction does not give 100% conversion. Since the E-factor is
simply the quotient kgwaste:kgproduct, everything which is not “product” is defined as “waste”.
1 Last updated March 31, 2008
Selected answers for exercises 2 www.catalysisbook.org
Thus, even a reactant which is left over (as in this example, because of incomplete
conversion) is "waste". In some cases, you can minimise this waste by feeding back your
unused reactant into the reactor, but this is not always the case, since it is not always so
easy to separate the reactants from the other byproducts (even assuming that you separate
the product). Sometimes (often, in the case of pharmaceuticals and fine chemicals) the “waste
reactant” is thrown away and a new reactant batch is used. An important point for the
students’ attention is that the E-factor uses kg, not mol. Chemistry students are sometimes
so used to using moles, they forget that the real world uses kg, because also when you
dispose of waste you pay for it by kg !!
Thus, for every two moles of C6H5Br and one mol of Cu, we obtain 0.85 moles of biphenyl and
0.85 moles of CuBr2. The overall reaction equation should be written as:
2C6H5Br + Cu → 0.85C6H5–C6H5 + 0.85CuBr2 + 0.15C6H5Br + 0.15Cu
Hopefully, we can recycle (part of) the surplus reagents. If not, we must count them as
“waste”. Thus, for every mol of biphenyl product, this reaction produces 1 mol of CuBr2 + 0.18
mol of C6H5Br + 0.18 mol of Cu. Since Mwbiphenyl = 154 gr/mol, every kg of biphenyl product
will give us
6.5 mol CuBr2 = 6.5 × (63.5+160) = 1452.75 gr CuBr2,
1.17 mol C6H5Br = 1.17 × (72+5+80) = 183.69 gr C6H5Br
1.17 mol Cu = 1.17 × 63.5 = 74.29 gr Cu
Total: 1453 + 187 + 74 = 1714 gr waste.
kg ( waste) 1.714
The overall E-factor is therefore: E factor = = = 1.714
kg ( product ) 1.000
Note that this E-factor is lower than the one above, even though we “threw away” 15% of the
reagents, because of the large difference in mass between iodine and bromine.
The corresponding Q-factors in both cases are medium.
(c) Calculating the catalyst TON and TOF. In this case, since the catalyst is a
macroporous solid, 5 wt% Pd/C, we can calculate the number of moles of Pd present in 100
mg catalyst (note: we can also refer directly to the metal weight (see also the discussion in
Chapter 4 on surface catalysis). 100 mg catalyst contains 5 mg Pd, and since MwPd = 106.4
gr/mol, we obtain 0.005/106.4 = 0.046 mmol Pd. If we assume that the substrate amount is 1
980mmol
mol, then after 40 minutes of reaction TON = = 21304 .
0.046mmol
The corresponding TOF is simply 21304/40 min = 532 min–1.
Note that the TON is always a pure number, while the TOF has units of time–1.
Background material on the web on E-factors and atom economy: The Green chemistry
network run by the Royal Society of Chemistry has an excellent website for education with
many links to good examples, see
http://www.chemsoc.org/networks/gcn/educate.htm
A1.5 (a) See the example of the analogous life-cycle diagram for milk cartons in Figure
1.25, p 33.
2 Last updated March 31, 2008
Selected answers for exercises 3 www.catalysisbook.org
(b) One fluorescent bulb consumes in its lifetime of 5000 h a total of 55 kWh. The
same 5000 h of operation require 5 incandescent bulbs, which in turn consume a total of 375
kWh, so the difference in favour of the fluorescent bulbs is 320 kWh. The thermal energy
content of coal is roughly 6.1 kWh/kg. However, although coal fired power generators are
very efficient, the amount of electricity generated per kg of coal is only 2.4 kWh. Thus, the
extra 320 kWh require an extra 133 kg of coal. A 20 ppm content of mercury corresponds to
20 mg mercury per kg coal, giving a total of 2660 mg mercury, of 2.66 gr mercury. Since a
typical fluorescent bulb contains less than 5 mg of mercury, fluorescent bulbs are much
better for the environment than incandescent ones.
Background material and further reading: There are several online articles on the “dim
threat” of mercury in fluorescent light bulbs, see for example the following investigation that
appeared on June 11, 2007, in Popular Mechanics:
http://www.popularmechanics.com/blogs/home_journal_news/4217864.html
A1.7 (For the definitions of TON and TOF, see p.11;)
When using p-chloro acetophenone, (R = Cl), 99% conversion was reached after 78 h, at a
substrate:catalyst ratio of 50 000 : 1. This means that each catalyst molecule is active on
average for 0.99 × 50 000, or 49 500 cycles. Thus:
TON = 49 500,
and TOF = 49 500/78 h = 635 h–1.
Similarly:
for R = CH3O, TON = 0.92 × 20 000 = 18 400, and TOF = 18 400 h–1.
and for R = F, TON = 1 × 500 = 500, and TOF = 500 h–1.
Note that in all these cases, the high conversions do not necessarily imply a high selectivity
to the desired product.
Selected answers for Chapter 2
A2.3 (a) Looking at the data in Table 2.2 (p. 71), we see that regardless of the starting
concentration, all of Michael’s reactions have reached 50% conversions after 120 min
(measurement 7 is a bad experiment and should be ignored). This points to a first-order
reaction. Moreover, the reaction is independent of the concentration of methane (since liquid
methane is the solvent, and the reaction volume is very large compared to the amount of
substrate). The corresponding rate equation is therefore: v = d[A]/dt = k[A].
(b) From Table 2.2, we know that t1/2 = ln2/k = 120 min. Solving for k we obtain:
k = ln2/t1/2 = ln2/120 min = 0.0057 min–1.
(c) Abdelhafid used much less methane, so it is not justified to say that the
concentration of methane does not change. A likely rate second-order rate equation that fits
the results of Abdelhafid is: v = d[A]/dt = k[A][CH4].
(d) The fact that Abdelhafid and Michael worked at different temperatures and using
different concentrations of methane accounts for the differences in their results. Michael
3 Last updated March 31, 2008
Selected answers for exercises 4 www.catalysisbook.org
used liquid methane as the solvent, transforming the second-order rate law into a pseudo-
first-order one. This means that the concentration of methane was so high that for all
practical purposes it did not change during the reaction.
(e) Both researchers would observe the deuterated lineatin as product. However,
changes in the reaction rates would be observed if the use of the deuterated substrate would
slow down one of the reaction steps so much so that it would become the new rate-
determining step. The magnitude of this slowing down would depend on whether the C–D
bond is broken in the rate-determining step or not. Such primary isotope effects are more
likely in the case of Abdelhafid’s experiments. Michael, on the other hand, may observe rate
changes due to solvent isotope effects.
A2.4 This is a first-order reaction, for which t1/2 = 20 min. Solving for k we obtain:
k = ln2/t1/2 = ln2/20 min = 0.0346 min–1.
The dependence of the conversion on time for a first-order reaction is given by:
– ln (1 – χA) = kt
We then substitute k = 0.0346 min–1 and χA = 0.999 (since we want the time at which the
reaction will reach 99.9% conversion), and obtain:
– ln (1 – 0.999) = 0.0346 × t
and therefore:
t = – ln(0.001)/0.0346 = 199.31 min ~ 200 min, so (b) is the only correct answer.
4 Last updated March 31, 2008
Selected answers for exercises 5 www.catalysisbook.org
Selected answers for Chapter 3
A3.2 (a,b) The figure below shows the completed catalytic cycle with the oxidation state of
the Pd atom in the various catalytic intermediates.
PdII complex
L Cu base-HBr
L Pd Br
Br
R C6H5
R C6H5
B CuBr
A transmetalation
oxidative addition
PdII complex
L
L = ligand L Pd C6H5
L Pd
L Pd0 complex R
C
reductive elimination
(c) In general, changing the concentration and/or type of reagent or catalytic
intermediate that participates in the rate-determining step will affect the reaction kinetics.
Thus, to determine, for example, if the oxidative addition step is rate-determining, you can
perform a series of reactions using different starting concentrations of aryl bromide and
compare their reaction profiles. Additionally, you can compare the reaction profiles of aryl
halides that differ only in their halide leaving group, such as {p-F-C6H4CH3, p-Cl-C6H4CH3,
p-Br-C6H4CH3, and p-I-C6H4CH3}.
Background material on the web: Check out Rob Toreki’s well presented organometallic
hypertextbook, see
http://www.ilpi.com/organomet/
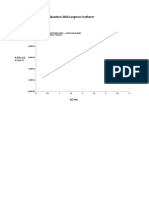
A3.4 (a) Looking at the graph below, we can see that there is a maximum of activity at a
2:1 pyridine:Cr ratio. This indicates the involvement of a complex containing one Cr atom
and two pyridine molecules (ligands) in the rate-determining step.
5 Last updated March 31, 2008
Selected answers for exercises 6 www.catalysisbook.org
900
832
800
700
650
600
TOF (1/min)
500
468
400
342
300
200
100 86
0
0 2 4 6 8 10
pyridine/CrCl3 ratio
(c) A possible mechanism involving free-radical intermediates is a chain reaction
mechanism with carene-oxo and carene-peroxo radicals carrying the chain. Since the
initiation step is largely independent of the organic residue of the hydroperoxide, and since
tert-butyl and carene have similar physical properties (e.g., their solubility in the reaction
medium is probably very similar) we can expect that adding 1 mol% of tert-butyl
hydroperoxide would give the same effect.
A3.6 Tolman’s cone angle is the base angle of a cone that encompasses the ligand, with the
metal centre in its apex and the ligating P atom (the cone angle is defined for Phosphorous
ligands, but can be in principle adapted to other ligating atoms) at a distance of 2.28
angstroms from it. The ligand bulk radius is the distance between the metal atom and the
bulkiest cross-section of the ligand. The ligand solid angle is the solid angle at this cross-
section.
In general, the cone angle is a good descriptor for C3-symmetric ligands, such as PPh3 and
P(o-tolyl)3, while the other two descriptors are useful in cases of asymmetric ligands and ones
with a centre of mass far from the ligating atoms. The best approach, however, is to calculate
all three parameters and then examine their correlation with the predicted and/or
experimental catalyst performance. For more details, see the discussion on catalyst
descriptors in Chapter 6, pp. 241–248.
6 Last updated March 31, 2008
Selected answers for exercises 7 www.catalysisbook.org
Selected answers for Chapter 4
Nafion
A4.1 (a) (CH3)2CH=CH2 + H2O → (CH3)3COH
(c) The excess of tert-butanol is added to enhance the solubility of isobutylene in the
reaction medium. In this case, adding n-pentanol would have a similar effect, since the two
alcohols have similar structures and solubility parameters.
A4.5 (a) This is a type IV isotherm, which corresponds to a mesoporous solid, so it cannot
be B, C, D, or G (for a discussion of isotherm types, see pp. 146–148). Neither the enzyme E
nor the polystyrene support F would survive the calcination (the enzyme would be denatured
even earlier, as the reaction temperature is 130 ºC). Catalyst A (Pt on mesoporous silica) is
the only correct answer.
(b) The surface area is the same before and after the 1st reaction (look at the first
part of the isotherm). However, the pore volume is smaller, so something must be ‘stuck’ in
the pores, for example oligomer/polymer molecules formed by side-reactions. Nothing drastic
has happened to the catalyst (i.e. the pores have not collapsed) as it evidently regained its
activity following the calcination.
(c) Looking just at the surface area would show no change, so it is a bad move to try
and save money in this case.
Background material on the web on adsorption isotherms: Wikipedia has a rather good
article that explains and gives examples of the isotherms of different materials. See
http://en.wikipedia.org/wiki/Adsorption
7 Last updated March 31, 2008
You might also like
- 9701 Nos Ps 5Document5 pages9701 Nos Ps 5Hubbak KhanNo ratings yet
- Limiting ReactantDocument10 pagesLimiting ReactantAimee NguyễnNo ratings yet
- Chapter 9 and 10Document22 pagesChapter 9 and 10Paolo GochingcoNo ratings yet
- Chapter 6 Review SolutionsDocument3 pagesChapter 6 Review SolutionshelloblargNo ratings yet
- Chemical Engineering Principles for Chemical ReactionsDocument6 pagesChemical Engineering Principles for Chemical ReactionsNicole Anne Borromeo0% (1)
- Chapter 9Document17 pagesChapter 9JajejijojuNo ratings yet
- Fourth Chapter - Part 2Document20 pagesFourth Chapter - Part 2toslim jahidNo ratings yet
- Chemical Formulas and Equations PDFDocument14 pagesChemical Formulas and Equations PDFJomarie Cabuello100% (1)
- Anaerobic Processes (Chapter 10) : AdvantagesDocument13 pagesAnaerobic Processes (Chapter 10) : AdvantagesΔημητρηςΣαρακυρουNo ratings yet
- Atom EconomyDocument27 pagesAtom Economycandysun100% (1)
- Chemical Reaction Stoichiometry and Material BalancesDocument30 pagesChemical Reaction Stoichiometry and Material BalancesMuhammad Azri HaziqNo ratings yet
- Che 311 PDFDocument7 pagesChe 311 PDFfamouscNo ratings yet
- Atom EconomyDocument21 pagesAtom EconomyJoel AriahuNo ratings yet
- Stoichiometry 2Document25 pagesStoichiometry 2Özlem GülcenNo ratings yet
- 1PGenChem Learning ModuleDocument7 pages1PGenChem Learning ModuleAngie ReblandoNo ratings yet
- LM - Stoichiometry Part 2 PDFDocument11 pagesLM - Stoichiometry Part 2 PDFikennahtNo ratings yet
- Ch. 10 PDFDocument20 pagesCh. 10 PDFDr.AhmedNo ratings yet
- Choosing A Basis: Chemical Engineering Principles - First Year/ Chapter Three Dr. Ahmed Faiq Al-AlawyDocument4 pagesChoosing A Basis: Chemical Engineering Principles - First Year/ Chapter Three Dr. Ahmed Faiq Al-AlawyMaheshree GohilNo ratings yet
- Chemical Reaction Rates and StoichiometryDocument53 pagesChemical Reaction Rates and Stoichiometryhanna bellanNo ratings yet
- Biogasification of Lignite Coal: Adi Krishna Print This Article Email This ArticleDocument25 pagesBiogasification of Lignite Coal: Adi Krishna Print This Article Email This ArticleSetyo Kf SariNo ratings yet
- Assignment ReactiveDocument2 pagesAssignment ReactiveNUREEN DAYANA BINTI MOHD IZMANIZAN A21ET01940% (1)
- Homework - Molecular ChemistryDocument5 pagesHomework - Molecular ChemistryJoana TolentinoNo ratings yet
- Chapter 4 - StudentDocument69 pagesChapter 4 - Studenteja70No ratings yet
- LE 005 007 General Chemistry 1 Continuation .Updated FinalDocument26 pagesLE 005 007 General Chemistry 1 Continuation .Updated FinalShaman KingNo ratings yet
- Reacciones y Extructuras LigninDocument23 pagesReacciones y Extructuras LigninHeyner Angulo PalacioNo ratings yet
- Percent Yield: Chemfile Mini-Guide To Problem SolvingDocument11 pagesPercent Yield: Chemfile Mini-Guide To Problem SolvingdhavaleshNo ratings yet
- chm420 Experiment 4Document11 pageschm420 Experiment 4Afeq NasemeNo ratings yet
- Set3ans 10Document5 pagesSet3ans 10amalinaishahNo ratings yet
- By J. Gutow 8/2007 Fuel ValuesDocument2 pagesBy J. Gutow 8/2007 Fuel ValuesMiriam TorreNo ratings yet
- Practical QuestionsDocument6 pagesPractical QuestionsLidiya MitikuNo ratings yet
- Mass Balance Tutorial 2 - 2021 Fin-StuDocument2 pagesMass Balance Tutorial 2 - 2021 Fin-StuToanique HeadmanNo ratings yet
- Chemical Stoichiometry CHEM 107Document43 pagesChemical Stoichiometry CHEM 107AhmedAdelIbrahimNo ratings yet
- Inorganic Catalysts Bronze Problem 2Document11 pagesInorganic Catalysts Bronze Problem 2joell2253h.01No ratings yet
- Stoichiometry Via Chemlog: Dr. Stephen Thompson Mr. Joe Staley Ms. Mary PeacockDocument14 pagesStoichiometry Via Chemlog: Dr. Stephen Thompson Mr. Joe Staley Ms. Mary PeacocksandrakristikNo ratings yet
- Orca Share Media1580335522780Document7 pagesOrca Share Media1580335522780elaine faithNo ratings yet
- Stoichiometry Solution Gen Chem 2 2Document36 pagesStoichiometry Solution Gen Chem 2 2Ura Angela FernandezNo ratings yet
- Unit 3 Review SolutionsDocument5 pagesUnit 3 Review SolutionshelloblargNo ratings yet
- Homework 1 EVF401G Material and Energy Balance: Due Date: 23:59 PM, Monday 17.01.2022Document17 pagesHomework 1 EVF401G Material and Energy Balance: Due Date: 23:59 PM, Monday 17.01.2022Sabrina RosazzaNo ratings yet
- Olimpiade Internasional Topik StoikiometriDocument7 pagesOlimpiade Internasional Topik StoikiometriHeru Christian Strecker AritonangNo ratings yet
- Chapter 2 Part 2Document56 pagesChapter 2 Part 2FATMIENo ratings yet
- Manual Experiment SMA TestDocument9 pagesManual Experiment SMA TestJair Ferreira JúniorNo ratings yet
- 07 Chemical ReactionDocument16 pages07 Chemical ReactionChrissa GuicoNo ratings yet
- Biochemistry 8th Edition Campbell Solutions Manual DownloadDocument8 pagesBiochemistry 8th Edition Campbell Solutions Manual DownloadClarence Lee100% (23)
- IbchstoichDocument11 pagesIbchstoichapi-293306937No ratings yet
- Eveg 4120 HW 9Document2 pagesEveg 4120 HW 9Mason SNo ratings yet
- Stoichiometry Lectures 4, 5, 6 and 7 CHE1010Document77 pagesStoichiometry Lectures 4, 5, 6 and 7 CHE1010Wayne MachinguraNo ratings yet
- Examples For Process SynthesisDocument14 pagesExamples For Process SynthesisgoldflackNo ratings yet
- Experiment 4: Limiting Reactant, Excess Reactant & Percent YieldDocument43 pagesExperiment 4: Limiting Reactant, Excess Reactant & Percent YieldAfrina FazrulNo ratings yet
- Week 7 Assignment QuestionsDocument4 pagesWeek 7 Assignment QuestionsTilakLNRangaNo ratings yet
- Chem 1 Week 4 Stoichiometry CompilerDocument7 pagesChem 1 Week 4 Stoichiometry CompilerMelcorr MontesclarosNo ratings yet
- STOICHIOMETRYDocument12 pagesSTOICHIOMETRYPheneloppe GarciaNo ratings yet
- Learn Mole ConceptsDocument4 pagesLearn Mole Conceptsshaikha_77No ratings yet
- Material Balances: Dr. M. ColeyDocument28 pagesMaterial Balances: Dr. M. ColeyToanique HeadmanNo ratings yet
- Hill Reaction2Document4 pagesHill Reaction2serrajNo ratings yet
- Essential Chemical Concepts Session IIDocument26 pagesEssential Chemical Concepts Session IIHamza QureshiNo ratings yet
- In CH O1999Document5 pagesIn CH O1999CorneliaNo ratings yet
- 07 ChemicalReactions 2bDocument15 pages07 ChemicalReactions 2bchewazableNo ratings yet
- General Chemistry 1: Self-Learning ModuleDocument12 pagesGeneral Chemistry 1: Self-Learning ModuleMykhaela Louize GumbanNo ratings yet
- Ozone Kinetics Question Part BDocument1 pageOzone Kinetics Question Part BkeatyNo ratings yet
- 2015 Rekening ExamDocument10 pages2015 Rekening ExamkeatyNo ratings yet
- Spectroscopy Andrew Knox (Mass Spec.) Short Questions 2014 No Mass Spec. Short Question This Year. Instead There Was 2 NMR/ NMR 2013Document1 pageSpectroscopy Andrew Knox (Mass Spec.) Short Questions 2014 No Mass Spec. Short Question This Year. Instead There Was 2 NMR/ NMR 2013keaty100% (1)
- EetdagboekDocument1 pageEetdagboekkeatyNo ratings yet
- Standard AdditionDocument2 pagesStandard AdditionkeatyNo ratings yet
- Question 2013 Langmuir Isotherm: P. (Kpa-1) / V. (Cm-3)Document2 pagesQuestion 2013 Langmuir Isotherm: P. (Kpa-1) / V. (Cm-3)keatyNo ratings yet
- Thermodynamics Short QuestionsDocument1 pageThermodynamics Short QuestionskeatyNo ratings yet
- Surface Chemistry Long QuestionsDocument6 pagesSurface Chemistry Long QuestionskeatyNo ratings yet
- Surface Chemistry Short QuestionsDocument1 pageSurface Chemistry Short QuestionskeatyNo ratings yet
- Atomic Spectroscopy Chapter 9 ProblemsDocument3 pagesAtomic Spectroscopy Chapter 9 ProblemskeatyNo ratings yet
- Kinetics Short QuestionsDocument2 pagesKinetics Short QuestionskeatyNo ratings yet
- Jack Treacy Spectroscopy Long QuestionsDocument3 pagesJack Treacy Spectroscopy Long QuestionskeatyNo ratings yet
- Chemical Technology Short - Question.Ann HopperDocument1 pageChemical Technology Short - Question.Ann HopperkeatyNo ratings yet
- Green Chemistry Q&A with Ann HopperDocument1 pageGreen Chemistry Q&A with Ann HopperkeatyNo ratings yet
- Ann Hopper Fluids QuestionsDocument1 pageAnn Hopper Fluids QuestionskeatyNo ratings yet
- Catalysis & Catalysts: Facts and Figures About CatalystsDocument88 pagesCatalysis & Catalysts: Facts and Figures About CatalystskeatyNo ratings yet
- AAS and AES Spectroscopy Exam QuestionsDocument2 pagesAAS and AES Spectroscopy Exam QuestionskeatyNo ratings yet
- CHEM3218 Pharmaceutical Separation Science Revision QuestionsDocument8 pagesCHEM3218 Pharmaceutical Separation Science Revision QuestionskeatyNo ratings yet
- Transport of Dangerous GoodsDocument16 pagesTransport of Dangerous Goodskeaty100% (1)
- Lecture 2 Analysis MethodsDocument26 pagesLecture 2 Analysis MethodskeatyNo ratings yet
- Dr. Andrew Knox's Contact DetailsDocument45 pagesDr. Andrew Knox's Contact DetailskeatyNo ratings yet
- CHEM3218 P.Behan-3Document66 pagesCHEM3218 P.Behan-3keatyNo ratings yet
- Chapter 3 Infrared SpectrophotometryDocument106 pagesChapter 3 Infrared SpectrophotometrykeatyNo ratings yet
- CHEM 3210 - Sample Numerical Problems and SolutionsDocument2 pagesCHEM 3210 - Sample Numerical Problems and SolutionskeatyNo ratings yet
- BSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andDocument4 pagesBSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andkeatyNo ratings yet
- BSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andDocument4 pagesBSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andkeatyNo ratings yet
- CHEM 3210 Review Questions 2015Document3 pagesCHEM 3210 Review Questions 2015keatyNo ratings yet
- Sadettin Ozturk Aug2009 UFRJDocument31 pagesSadettin Ozturk Aug2009 UFRJkeatyNo ratings yet
- Bio Pharma 2015 - Profile PDFDocument76 pagesBio Pharma 2015 - Profile PDFmarvelousgraceNo ratings yet
- Root Cause Analysis Continuous AssessmentDocument2 pagesRoot Cause Analysis Continuous AssessmentkeatyNo ratings yet
- Hajj A Practical Guide-Imam TahirDocument86 pagesHajj A Practical Guide-Imam TahirMateen YousufNo ratings yet
- Grade 8 Lily ExamDocument3 pagesGrade 8 Lily ExamApril DingalNo ratings yet
- Sonigra Manav Report Finle-Converted EDITEDDocument50 pagesSonigra Manav Report Finle-Converted EDITEDDABHI PARTHNo ratings yet
- Agilent Cool On-Column Operation ManualDocument42 pagesAgilent Cool On-Column Operation Manualdmcevoy1965No ratings yet
- Prof. Ed - Assessment and Evaluation of Learning Part 1-4Document8 pagesProf. Ed - Assessment and Evaluation of Learning Part 1-4Marisol Altobar PalatinoNo ratings yet
- Abbreviation Meaning Notes: Cibo ("With Food")Document4 pagesAbbreviation Meaning Notes: Cibo ("With Food")TantriNo ratings yet
- One - Pager - SOGEVAC SV 320 BDocument2 pagesOne - Pager - SOGEVAC SV 320 BEOLOS COMPRESSORS LTDNo ratings yet
- Banu Maaruf of The LevantDocument6 pagesBanu Maaruf of The LevantMotiwala AbbasNo ratings yet
- Phaseo Abl7 Abl8 Abl8rps24100Document9 pagesPhaseo Abl7 Abl8 Abl8rps24100Magda DiazNo ratings yet
- DLL Grade7 First 1solutions ConcentrationDocument5 pagesDLL Grade7 First 1solutions ConcentrationJaneth de JuanNo ratings yet
- Chronological OrderDocument5 pagesChronological OrderDharWin d'Wing-Wing d'AriestBoyzNo ratings yet
- Solutions: Spheres, Cones and CylindersDocument13 pagesSolutions: Spheres, Cones and CylindersKeri-ann MillarNo ratings yet
- 1571-1635319494618-Unit 04 Leadership and ManagementDocument48 pages1571-1635319494618-Unit 04 Leadership and ManagementdevindiNo ratings yet
- European Business in China Position Paper 2017 2018 (English Version)Document408 pagesEuropean Business in China Position Paper 2017 2018 (English Version)Prasanth RajuNo ratings yet
- British Isles Composition GuideDocument4 pagesBritish Isles Composition GuidesonmatanalizNo ratings yet
- Act 1&2 and SAQ No - LawDocument4 pagesAct 1&2 and SAQ No - LawBududut BurnikNo ratings yet
- Internal Resistance and Matching in Voltage SourceDocument8 pagesInternal Resistance and Matching in Voltage SourceAsif Rasheed Rajput100% (1)
- Mad LabDocument66 pagesMad LabBalamurugan MNo ratings yet
- 3.1 C 4.5 Algorithm-19Document10 pages3.1 C 4.5 Algorithm-19nayan jainNo ratings yet
- Vincent Ira B. Perez: Barangay Gulod, Calatagan, BatangasDocument3 pagesVincent Ira B. Perez: Barangay Gulod, Calatagan, BatangasJohn Ramsel Boter IINo ratings yet
- 2 Druid StreetDocument4 pages2 Druid StreetthamestunnelNo ratings yet
- Ketchikan Shipyard Improvements Plan CompleteDocument230 pagesKetchikan Shipyard Improvements Plan CompleteOpó Ishak Bawias Adare100% (1)
- Circuit AnalysisDocument98 pagesCircuit Analysisahtisham shahNo ratings yet
- 96 Amazing Social Media Statistics and FactsDocument19 pages96 Amazing Social Media Statistics and FactsKatie O'BrienNo ratings yet
- Athenaze 1 Chapter 7a Jun 18th 2145Document3 pagesAthenaze 1 Chapter 7a Jun 18th 2145maverickpussNo ratings yet
- Aos Warscroll Fimir WarriorsDocument1 pageAos Warscroll Fimir WarriorsGuido Sebastián AlvarezNo ratings yet
- A ULTIMA ReleaseNotesAxiomV PDFDocument38 pagesA ULTIMA ReleaseNotesAxiomV PDFIVANALTAMARNo ratings yet
- Service Parts List: 54-26-0005 2551-20 M12™ FUEL™ SURGE™ 1/4" Hex Hydraulic Driver K42ADocument2 pagesService Parts List: 54-26-0005 2551-20 M12™ FUEL™ SURGE™ 1/4" Hex Hydraulic Driver K42AAmjad AlQasrawi100% (1)
- Timber, PVCu and aluminium window and door hardware systemsDocument24 pagesTimber, PVCu and aluminium window and door hardware systemsOmul Fara NumeNo ratings yet
- InteliLite AMF20-25Document2 pagesInteliLite AMF20-25albertooliveira100% (2)