Professional Documents
Culture Documents
Case Report: Anaplastic Meningioma: A Case Report and Literature Review
Uploaded by
prabowoaji12Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Case Report: Anaplastic Meningioma: A Case Report and Literature Review
Uploaded by
prabowoaji12Copyright:
Available Formats
Int J Clin Exp Med 2019;12(7):9418-9424
www.ijcem.com /ISSN:1940-5901/IJCEM0093615
Case Report
Anaplastic meningioma: a case report
and literature review
Chunping Mao1, Jun Li1, Lan Rao2
Departments of 1Radiology and 2Pathology, The Jingmen No. 1 People’s Hospital, Jingmen, Hubei, P.R. China
Received March 10, 2019; Accepted May 13, 2019; Epub July 15, 2019; Published July 30, 2019
Abstract: The aim of the present case study was to investigate the imaging manifestations, histopathological and
immunohistochemical characteristics of anaplastic meningioma (AM). We analyzed and summarized the charac-
teristics of AM in a single case using computed tomography (CT) and magnetic resonance imaging (MRI), histo-
pathological and immunohistochemical examinations. Imaging results of CT and MRI in the present case indicated
bone destruction of the left parietal bone with surrounding fusiform soft tissue mass, mild edema of the adjacent
brain tissue and compression of the adjacent venous sinus. On postcontrast T1-weighted MRI, the lesion exhibited
marked inhomogeneous enhancement and a dural tail sign. Pathological examination identified the lesion as AM
originating from the dura mater, and positive for epithelial membrane antigen and vimentin. Most AM lesions ap-
pear on brain imaging as cystic-solid masses, with an irregular shape and blurred margin, but when AM invades the
adjacent bone and causes bone destruction, it is essential for radiologists to differentiate it from other extracranial
tumors. CT and MRI examination are effective diagnostic methods for identifying AM preoperatively given that the
uncommon imaging manifestations of AM are well understood.
Keywords: Brain, anaplastic meningioma, recurrence, magnetic resonance imaging, tomography
Introduction scan revealed a fusiform inhomogeneous soft
tissue mass with bone destruction of the left
Anaplastic meningioma (AM) is a highly malig- parietal bone (Figure 1A-D). The radiologist
nant tumor classified as a grade III meningio- considered the possibility of a bone tumor, and
ma according to the 2016 World Health Or- the patient was referred for further investiga-
ganization (WHO) classification of tumors of the tion. A brain MRI revealed a space-occupying
central nervous system [1]. Although AM has a lesion with mixed intensity and a multiple cystic
high recurrence rate and an exceptionally poor signal pattern, unclear margins, with mild peri-
prognosis, it occurs rarely, accounting for only tumoral brain edema (Figure 2A-C). A magnetic
1%-2% of all meningiomas [2]. CT and MR ex- resonance venogram (MRV) revealed that the
aminations are usually able to diagnose typical adjacent superior sagittal sinus was slightly
AM cases, but when AM invades the surround- compressed (Figure 2D). Gadopentetate dime-
ing structures and causes corresponding ch- glumine- (Gd-DTPA) enhanced MRI demonstrat-
anges, diagnosis becomes more difficult and
ed marked inhomogeneous enhancement of
misdiagnosis may occur. The purpose of this
the lesion, with the dural tail sign (Figure 2E,
case report is to report atypical or rare imaging
2F). Those manifestations highlighted on CT
features of AM.
and MRI led to the diagnosis of eosinophilic
Case report granuloma or plasmacytoma of the left parietal
bone. Following completion of routine examina-
In May 2016, a 27-year-old man presented to tions, the patient underwent surgery. Simpson
The Jingmen No. 1 People’s Hospital (Jingmen, grade IV surgical resection was performed due
China), due to a mass on the left side of the to the poorly defined boundary between the
head detected incidentally one month earlier; tumor and adjacent tissue. Pathological exami-
the patient was asymptomatic. The brain CT nation revealed that the tumor was highly cel-
Mao et al: Anaplastic meningioma
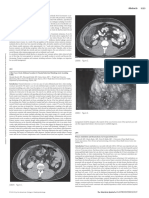
Figure 1. Brain CT showed the lesion to be located on the left top of the head, seen as a fusiform inhomogeneous
soft tissue mass with osteolytic destruction of the left parietal bone.
lular, with cells exhibiting prominent and over- temporal area, which indicated AM recurrence
lapping nucleoli, or multinucleated vesicular (Figure 5A). The patient then underwent a se-
cells (Figure 3). Immunohistochemistry results cond surgery of the left temporal meninges,
revealed that the tumor cells were weakly posi- and AM recurrence was confirmed (Figure 5B).
tive for epithelial membrane antigen (EMA) Over the next two months, the patient contin-
(Figure 4A), diffusely positive for vimentin (Fi- ued to undergo radiotherapy and received a
gure 4B), with patchy staining for progesterone course of chemotherapy (temozolomide, 250
receptor (PR) (Figure 4C), and a Ki-67 index of mg, once a day for five days), with unsatisfac-
20% (Figure 4D). Taken together, these results tory results. The last MRI scan in April 2017
led to the diagnosis of AM of the left parietal
indicated metastasis of the left parapharyn-
region.
geal space involving the adjacent bone and
Following surgery, the patient received radio- muscle tissue (Figure 6). The patient then
therapy, with GTV 64Gy/30F and CTV 56Gy/30F underwent seven courses of local palliative
five times a week. After 28 courses of radio- radiotherapy (GTV, 30gy/10F), with unsatisfac-
therapy, the first re-examination with Gd-DTPA- tory results and many complications. The
enhanced MRI demonstrated thickening and patient died in September 2017. Survival time
marked enhancement of the meninges in left after the initial diagnosis was 15 months.
9419 Int J Clin Exp Med 2019;12(7):9418-9424
Mao et al: Anaplastic meningioma
Figure 2. Brain MRI revealed that the lesion was heterogeneous with a long signal on T1-weighted images (B and
C) and T2-weighted imaging (A) with small cystic longer T1-weighted imaging and T2-weighted imaging areas. MRV
(D) revealed compression of the adjacent superior sagittal sinus by the lesion. Gd-DTPA-enhanced MRI (E and F)
revealed marked inhomogeneous enhancement of the lesion, with the dural tail sign.
Figure 3. Hematoxylin and eosin staining revealed that the tumor was highly cellular, with large cells exhibiting
prominent and overlapping nucleoli, or multinucleated vesicular cells. Magnification, ×40 (A) ×400 (B).
Discussion grade III has three variants, namely anaplastic,
rhabdoid and papillary. AM is the most com-
Meningioma is the most common intracranial mon grade III type, which has a high degree
brain tumor, accounting for over one-third of of malignancy and a high recurrence rate. The
primary brain neoplasms [3]. Meningioma is age at onset of AM ranges from 18 to 70 years
divided into 15 subtypes and 3 grades [1]; old [4]. Female predominance is noted only in
9420 Int J Clin Exp Med 2019;12(7):9418-9424
Mao et al: Anaplastic meningioma
Figure 4. On immunohistochemical staining the tumor was (A) focally positive for epithelial membrane antigen and
(B) diffusely positive for vimentin. (C) the staining for progesterone receptor was patchy and (D) the Ki-67 index was
~20%. Magnification, ×400.
Figure 5. Postoperatively, the patient underwent the first reexamination in June 2016. (A) Gd-DTPA-enhanced MRI
revealed thickening of the meninges in left temporal area with obvious enhancement (arrow). (B) After the second
surgery, hematoxylin and eosin staining of the left temporal meninges revealed that the tumor was metastasis of
AM. Magnification, ×400.
9421 Int J Clin Exp Med 2019;12(7):9418-9424
Mao et al: Anaplastic meningioma
Figure 6. The last enhanced MRI scan in April 2017 indicated metastasis of the left parapharyngeal space involv-
ing the adjacent bone and muscle tissue (A, short arrow). Partial absence of left temporal bone after the second
surgery (B, long arrow).
benign meningioma and male predominance AM may exhibit various appearances on CT and
is found in atypical and malignant variants [5]. MRI but may also share some common charac-
The clinical characteristics of AM are not ty- teristics. Most AM cases have a wide tumor
pical, most patients present with signs and base and are usually lobulated or irregular fusi-
symptoms attributable to mass effect at the form in shape as in the present case. On con-
tumor site, including headache, seizure, and ventional CT scan, AM appears as a low-density
hemiparesis, while some patients are asymp- shadow with areas of cystic degeneration and
tomatic [6]. The locations of AM include con- calcification. On conventional MRI, the tumor is
vexity dura mater, skull base, tentorium, falx, or iso- to hypointense on T1-weighted imaging and
intraventricular space [7, 8]. Variable reports of iso- to hyperintense on T2-weighted imaging
median overall survival are found in the litera- [12]. Both CT and MRI scans demonstrated
ture, with some series reporting a survival of that the tumor in our case had an unclear
1.5-3.5 years, a 5-year survival rate of 35%- boundary. Mild to severe peritumoral brain
61% [9-11], which may be due to variations in edema was seen, which was attributed to com-
the times of distant metastasis. A retrospec- pression of the adjacent venous sinuses and
tive study demonstrated that the factors asso- tumor invasion of the surrounding brain tis-
ciated with the progression-free survival of AM sue. On contrast-enhanced CT or MRI, the AM
were preoperative Karnofsky performance sta- appeared to have significant inhomogeneity
tus, extent of tumor resection, radiotherapy, enhancement with the dural tail sign. Of note,
tumor location and a history of meningioma although the dural tail sign is specific for menin-
[12]. Approximately 80% of patients with recur- gioma, it is not unique to meningioma. It may
rence develop tumor regrowth and metastasis, also indicate invading tumor cells, hyperplastic
but extracranial metastases in these cases is fibrous connective tissue, and abundant and
rare, accounting for only 0.1%. Although sur- expansive blood vessels [15].
gery is considered the primary treatment of
choice for such cases, the high recurrence and CT and MRI have specific advantages in the
metastasis rates require other adjuvant thera- diagnosis of AM: CT scan is used mainly to eval-
peutic modalities, such as external beam radio- uate bone invasion, and MRI to evaluate the
therapy [6, 13]. Various targeted chemothera- relationship between the tumor and adjacent
pies such as somatostatin analogues are ac- structures. However, when the AM causes adja-
tively under investigation [14]. cent bone destruction, as in the present case,
9422 Int J Clin Exp Med 2019;12(7):9418-9424
Mao et al: Anaplastic meningioma
differentiating it from bone tumors may be dif- The patient provided signed information con-
ficult. It may be helpful to analyze the relation- sent for this case report to be produced. The
ship between mass and bone destruction; the investigation for this case report was approv-
geometric center of the bone destruction and ed by the Medical Ethics Committee of The
the mass are asymmetric, and this characteris- Jingmen No. 1 People’s Hospital.
tic can be useful in the differential diagnosis
of AM from eosinophilic granuloma and plas- Acknowledgements
macytoma. Due to the exceptionally high recur-
rence and metastatic rates of AM, enhanced We would like to thank Dr. Qiubo Li who provid-
MRI is recommended for routine postoperative ed clinical information.
check-ups.
Disclosure of conflict of interest
The diagnosis of AM relies on the presence of
None.
morphological characteristics and immunohis-
tochemical markers expressed in the tumor Address correspondence to: Dr. Chunping Mao,
cells. The gross specimen of AM is greyish red. Department of Radiology, The Jingmen No. 1 Peo-
Under the microscope, the tumor either exhib- ple’s Hospital, 168 Xiangshan Road, Jingmen
its malignant cytology (carcinoma-, sarcoma-, 448000, Hubei, P.R. China. Tel: 15717829848;
or melanoma-like histology), or a markedly ele- E-mail: dr_maochunping@126.com
vated mitotic index (≥20 mitoses per 10 high-
power fields), without papillary architecture or References
rhabdoid cytology, and >1 per high-power field
[16, 17]. On immunohistochemistry analysis, [1] Louis DN, Perry A, Reifenberger G, von Deimling
like meningiomas of all grades, patchy positive A, Figarella-Branger D, Cavenee WK, Ohgaki H,
Wiestler OD, Kleihues P and Ellison DW. The
immunoreactivity for EMA will be noted in AM,
2016 world health organization classification
and some immunopositivity for vimentin [18]. of tumors of the central nervous system: a
Together these characteristic results suggest summary. Acta Neuropathol 2016; 131: 803-
that the tumor originates from the arachnoid 820.
membrane. PR tends to be negative or with a [2] Orton A, Frandsen J, Jensen R, Shrieve DC and
patchy positive pattern in AM compared with Suneja G. Anaplastic meningioma: an analysis
lower grade meningiomas [19]. S-100 is a of the national cancer database from 2004 to
marker for epidermal differentiation, which is 2012. J Neurosurg 2018; 128: 1684-1689.
mainly used for the identification of meningio- [3] Ostrom QT, Gittleman H, Farah P, Ondracek A,
ma and schwannoma but is less expressed in Chen Y, Wolinsky Y, Stroup NE, Kruchko C and
Barnholtz-Sloan JS. CBTRUS statistical report:
AM [20]. Recently, somatostatin receptor 2a
Primary brain and central nervous system tu-
has emerged as a highly sensitive and specific mors diagnosed in the United States in 2006-
diagnostic marker for meningiomas of all gr- 2010. Neuro-oncol 2013; 15 Suppl 2: ii1-ii56.
ades, and appears to be superior to EMA and [4] Aizer AA, Bi WL, Kandola MS, Lee EQ, Nayak L,
PR, particularly in cases of AM [21]. Rinne ML, Norden AD, Beroukhim R, Reardon
DA, Wen PY, Al-Mefty O, Arvold ND, Dunn IF and
In conclusion, since AM is prone to metastasis Alexander BM. Extent of resection and overall
and recurrence, it represents a major challenge survival for patients with atypical and malig-
in terms of treatment making accurate diagno- nant meningioma. Cancer 2015; 121: 4376-
sis essential. AM exhibits the general imaging 4381.
characteristics of malignant tumors, but when [5] Cornelius JF, Slotty PJ, Steiger HJ, Hänggi D,
it causes bone destruction, observing whether Polivka M and George B. Malignant potential
symmetry exists between bone destruction and of skull base versus non-skull base meningi-
omas: Clinical series of 1,663 cases. Acta
mass may provide clues to the diagnosis. AM
Neurochir (Wien) 2013; 155: 407-413.
can be accurately diagnosed through a combi- [6] Sughrue ME, Sanai N, Shangari G, Parsa AT,
nation of imaging and pathological findings. Berger MS and McDermott MW. Outcome and
From a radiological point of view, imaging can survival following primary and repeat surgery
help with the timely identification of metastasis for World Health Organization Grade III menin-
following tumor resection and radiotherapy. giomas. J Neurosurg 2010; 113: 202-209.
9423 Int J Clin Exp Med 2019;12(7):9418-9424
Mao et al: Anaplastic meningioma
[7] Durand A, Labrousse F, Jouvet A, Bauchet L, [15] Hutzelmann A, Palmié S, Buhl R, Freund M and
Kalamaridès M, Menei P, Deruty R, Moreau JJ, Heller M. Dural invasion of meningiomas
Fèvre-Montange M and Guyotat J. WHO grade adjacent to the tumor margin on Gd-DTPA-
II and III meningiomas: a study of prognostic enhanced MR images: histopathologic correla-
factors. J Neurooncol 2009; 95: 367-375. tion. Eur Radiol 1998; 8: 746-748.
[8] Kane AJ, Sughrue ME, Rutkowski MJ, Shangari [16] Hanft S, Canoll P������������������������������
and��������������������������
Bruce JN. A review of ma-
G, Fang S, McDermott MW, Berger MS and lignant meningiomas: diagnosis, characteris-
Parsa AT. Anatomic location is a risk factor for tics, and treatment. J Neuro-oncol 2010; 99:
atypical and malignant meningiomas. Cancer 433-443.
2011; 117: 1272-1278. [17] Cimino PJ: Malignant progression to anaplastic
[9] Adeberg S, Hartmann C, Welzel T, Rieken S, meningioma: neuropathology, molecular pa-
Habermehl D, von Deimling A, Debus J and thology, and experimental models. Exp Mol
Combs SE. Long-term outcome after radiother- Pathol 2015; 99: 354-359.
apy in patients with atypical and malignant [18] Liu Y, Sturgis CD, Bunker M, Saad RS, Tung
meningiomas--clinical results in 85 patients M, Raab SS and Silverman JF. Expression
treated in a single institution leading to opti- of cytokeratin by malignant meningiomas:
mized guidelines for early radiation therapy. Int Diagnostic pitfall of cytokeratin to separate
J Radiat Oncol Biol Phys 2012; 83: 859-864. malignant meningiomas from metastatic carci-
[10] Champeaux C, Wilson E, Brandner S, Shieff C noma. Mod Pathol 2004; 17: 1129-1133.
and Thorne L. World Health Organization grade [19] Perry A, Cai DX, Scheithauer BW, Swanson
III meningiomas. A retrospective study for out- PE, Lohse CM, Newsham IF, Weaver A and
come and prognostic factors assessment. Br J Gutmann DH. Merlin, DAL-1, and progesterone
Neurosurg 2015; 29: 693-698. receptor expression in clinicopathologic sub-
[11] Moliterno J, Cope WP, Vartanian ED, Reiner AS, sets of meningioma: a correlative immunohis-
Kellen R, Ogilvie SQ, Huse JT and Gutin PH. tochemical study of 175 cases. J Neuropathol
Survival in patients treated for anaplastic men- Exp Neurol 2000; 59: 872-879.
ingioma. J Neurosurg 2015; 123: 23-30. [20] Ng J, Celebre A, Munoz DG, Keith JL and
[12] Zhu H, Xie Q, Zhou Y, Chen H, Mao Y, Zhong P, Karamchandani JR. Sox10 is superior to
Zheng K, Wang Y, Wang Y, Xie L, Zheng M, Tang S100 in the diagnosis of meningioma. Appl
H, Wang D, Chen X, Zhou L and Gong Y. Analysis Immunohistochem Mol Morphol 2015; 23:
of prognostic factors and treatment of ana- 215-219.
plastic meningioma in China. J Clin Neurosci [21] Menke JR, Raleigh DR, Gown AM, Thomas S,
2015; 22: 690-695. Perry A and Tihan T. Somatostatin receptor 2a
[13] Sun SQ, Hawasli AH, Huang J, Chicoine MR and is a more sensitive diagnostic marker of men-
Kim AH. An evidence-based treatment algo- ingioma than epithelial membrane antigen.
rithm for the management of WHO Grade II Acta Neuropathol 2015; 130: 441-443.
and III meningiomas. Neurosurg Focus 2015;
38: E3.
[14] Norden AD, Ligon KL, Hammond SN, Muzi-
kansky A, Reardon DA, Kaley TJ, Batchelor TT,
Plotkin SR, Raizer JJ, Wong ET, Drappatz J,
Lesser GJ, Haidar S, Beroukhim R, Lee EQ,
Doherty L, Lafrankie D, Gaffey SC, Gerard M,
Smith KH, McCluskey C, Phuphanich S and
Wen PY. Phase II study of monthly pasireotide
LAR (SOM230C) for recurrent or progressive
meningioma. Neurology 2015; 84: 280-286.
9424 Int J Clin Exp Med 2019;12(7):9418-9424
You might also like
- Unit Plan For Sem 1Document17 pagesUnit Plan For Sem 1MRS CHAKRAPANI100% (10)
- Chap004 Test Bank Tank PDFDocument53 pagesChap004 Test Bank Tank PDFHeraNo ratings yet
- Sesi 6-Fisiologi Neuromuskuler IDocument86 pagesSesi 6-Fisiologi Neuromuskuler IGiselleNo ratings yet
- tmpBAE8 TMPDocument6 pagestmpBAE8 TMPFrontiersNo ratings yet
- Third Ventricular Papillary MeningiomaDocument4 pagesThird Ventricular Papillary MeningiomaArif Susilo RahadiNo ratings yet
- 17 OmerhodzicDocument9 pages17 OmerhodzicFederico MinghinelliNo ratings yet
- 1208 Case 1Document2 pages1208 Case 1willygopeNo ratings yet
- 0610case1 Metastatic MelanomaDocument4 pages0610case1 Metastatic MelanomawillygopeNo ratings yet
- New Approaches For The Treatment of Refractory Meningiomas: Brian Ragel, MD, and Randy L. Jensen, MD, PHDDocument11 pagesNew Approaches For The Treatment of Refractory Meningiomas: Brian Ragel, MD, and Randy L. Jensen, MD, PHDAnonymous KKqrboTNo ratings yet
- Translated Laporan Kasus Meningioma Spinal en Plaque IDEM Dengan KalsifikasiDocument7 pagesTranslated Laporan Kasus Meningioma Spinal en Plaque IDEM Dengan KalsifikasiArif Susilo RahadiNo ratings yet
- Neoadjuvant Chemotherapy For Primary Sarcoma of The Breast: A Case ReportDocument6 pagesNeoadjuvant Chemotherapy For Primary Sarcoma of The Breast: A Case ReportRicky Aris FandikaNo ratings yet
- Ajnr Benign Enhancing Lesion of Foramen MagnumDocument5 pagesAjnr Benign Enhancing Lesion of Foramen Magnumapi-288086961No ratings yet
- Article in Press: Journal of Oral and Maxillofacial Surgery, Medicine, and PathologyDocument6 pagesArticle in Press: Journal of Oral and Maxillofacial Surgery, Medicine, and PathologyOMFS FKG UnimusNo ratings yet
- Intracranial Yolk Sac Tumor in An Adult Patient: MRI, Diffusion-Weighted Imaging and H MR Spectroscopy FeaturesDocument4 pagesIntracranial Yolk Sac Tumor in An Adult Patient: MRI, Diffusion-Weighted Imaging and H MR Spectroscopy FeaturestiaraNo ratings yet
- Rapid Recurrence of A Malignant Meningioma Case ReDocument4 pagesRapid Recurrence of A Malignant Meningioma Case Revivi talawoNo ratings yet
- Angiofibroma GK BuffaloDocument5 pagesAngiofibroma GK BuffaloZdravko HeinrichNo ratings yet
- Multiple MeningiomasDocument3 pagesMultiple Meningiomasarun gowdaNo ratings yet
- Meu Artigo MeningiomaDocument8 pagesMeu Artigo MeningiomapeddrohNo ratings yet
- Magnetic Resonance Imaging of Meningiomas: A Pictorial ReviewDocument10 pagesMagnetic Resonance Imaging of Meningiomas: A Pictorial ReviewKevin EdroNo ratings yet
- Malignent MelanomaDocument41 pagesMalignent MelanomashokoNo ratings yet
- Imaging Characteristics and Surgical Treatment of Invasive MeningiomaDocument6 pagesImaging Characteristics and Surgical Treatment of Invasive Meningiomaniluhputu asrinidewiNo ratings yet
- Giant Cell Tumor of Tendon Sheath YjfasDocument6 pagesGiant Cell Tumor of Tendon Sheath YjfasPaulo Victor Dias AlmeidaNo ratings yet
- Orbital Metastasis of A Pediatric Nasopharyngeal Carcinoma A Rare Case ReportDocument4 pagesOrbital Metastasis of A Pediatric Nasopharyngeal Carcinoma A Rare Case ReportInternational Journal of Innovative Science and Research Technology100% (1)
- ONCO 21 Desember 2020 Undiferentiated Pleomorfic Sarcoma Rev 1Document46 pagesONCO 21 Desember 2020 Undiferentiated Pleomorfic Sarcoma Rev 1ciptakarwanaNo ratings yet
- MeningiomaDocument4 pagesMeningiomaHariyehNo ratings yet
- Metastatic Vertebral Lesion Mimicking An Atypical Hemangioma WithDocument8 pagesMetastatic Vertebral Lesion Mimicking An Atypical Hemangioma Withsica_17_steaua6519No ratings yet
- Imagerie Des TumeursDocument14 pagesImagerie Des TumeursOuma TaziNo ratings yet
- Meningioma With Cystic Change Mimicking Hemangioblastoma: SciencedirectDocument5 pagesMeningioma With Cystic Change Mimicking Hemangioblastoma: SciencedirectKhương Hà NguyễnNo ratings yet
- Follow UpDocument6 pagesFollow UpWinda HaeriyokoNo ratings yet
- Calcified Pseudoneoplasm of The Neuraxis Capnon A Lesson Learnt From A Rare Entity PDFDocument3 pagesCalcified Pseudoneoplasm of The Neuraxis Capnon A Lesson Learnt From A Rare Entity PDFKisspetre PetreNo ratings yet
- TMP BCFFDocument3 pagesTMP BCFFFrontiersNo ratings yet
- Extracranial Metastases of Anaplastic Meningioma: Case ReportDocument6 pagesExtracranial Metastases of Anaplastic Meningioma: Case ReportRizky AdriansahNo ratings yet
- GCT ThumbDocument10 pagesGCT ThumbMoeez AkramNo ratings yet
- Giant Cell Tumor of The Phalanx of Finger: Case Reports: BackgroundDocument10 pagesGiant Cell Tumor of The Phalanx of Finger: Case Reports: BackgroundMoeez AkramNo ratings yet
- Yuh2009 Article ImagingOfEpendymomasMRIAndCTDocument11 pagesYuh2009 Article ImagingOfEpendymomasMRIAndCTHuyen NguyenNo ratings yet
- Osteosarcoma of The Jaw: Report of 3 Cases (Including The Rare Epithelioid Variant) With Review of LiteratureDocument10 pagesOsteosarcoma of The Jaw: Report of 3 Cases (Including The Rare Epithelioid Variant) With Review of LiteraturesTEVENNo ratings yet
- Imaging in Brain MeningiomaDocument12 pagesImaging in Brain MeningiomaBramanda Sml TobingNo ratings yet
- Deep Sylvian Meningioma: A Case Report and Review of LiteratureDocument4 pagesDeep Sylvian Meningioma: A Case Report and Review of LiteratureLINA ESTRADA DUQUENo ratings yet
- Combined Modalities of Surgery, Radiotherapy, Radiosurgery and Chemotherapy For Invasive Pituitary CarcinomaDocument4 pagesCombined Modalities of Surgery, Radiotherapy, Radiosurgery and Chemotherapy For Invasive Pituitary CarcinomaTony Miguel Saba SabaNo ratings yet
- Chondrosarcoma of The Temporomandibular Joint: A Case Report and Review of The LiteratureDocument9 pagesChondrosarcoma of The Temporomandibular Joint: A Case Report and Review of The LiteratureCatalina Soler LioiNo ratings yet
- Ajol File Journals - 449 - Articles - 116044 - Submission - Proof - 116044 5329 322635 1 10 20150421Document6 pagesAjol File Journals - 449 - Articles - 116044 - Submission - Proof - 116044 5329 322635 1 10 20150421valeria navarroNo ratings yet
- Mixed Malignant Germ Cell Tumour of The Lateral Ventricle in An 8-Month-Old Girl: Case Report and Review of The LiteratureDocument4 pagesMixed Malignant Germ Cell Tumour of The Lateral Ventricle in An 8-Month-Old Girl: Case Report and Review of The LiteratureEslam KandilNo ratings yet
- A Case of Malignant Pleural Mesothelioma With Metastasis To The OrbitDocument3 pagesA Case of Malignant Pleural Mesothelioma With Metastasis To The OrbitjamesyuNo ratings yet
- KLP 10 - Jurnal - MeningiomaDocument11 pagesKLP 10 - Jurnal - Meningiomawahy udiNo ratings yet
- Radiology of Acute Mastoiditis and Its Complications: A Pictorial Review and Interpretation ChecklistDocument6 pagesRadiology of Acute Mastoiditis and Its Complications: A Pictorial Review and Interpretation ChecklistSalma Nurisna UfairohNo ratings yet
- Primary Ameloblastic Carcinoma of The Maxilla A CaDocument9 pagesPrimary Ameloblastic Carcinoma of The Maxilla A CaMuhammad Avicenna AdjiNo ratings yet
- Meningioma Quístico, Un Desafío DiagnósticoDocument4 pagesMeningioma Quístico, Un Desafío DiagnósticoPamela WheelockNo ratings yet
- AsianJNeurosurg102126-4310714 115827 PDFDocument3 pagesAsianJNeurosurg102126-4310714 115827 PDFSucipto HartonoNo ratings yet
- Myeloma MultipleDocument7 pagesMyeloma MultipleCriissthiiann HernnandezNo ratings yet
- Primary Leiomyosarcoma of The Spine: An Analysis of Imaging Manifestations and Clinicopathological FindingsDocument9 pagesPrimary Leiomyosarcoma of The Spine: An Analysis of Imaging Manifestations and Clinicopathological Findingst7xs5mchfzNo ratings yet
- Isolated Lesser Trochanter Fracture Associated With LeukemiaDocument3 pagesIsolated Lesser Trochanter Fracture Associated With LeukemiaIndora DenniNo ratings yet
- Romano Et Al 2020 Solitary Fibrous Tumor of The Deep Parotid GlandDocument3 pagesRomano Et Al 2020 Solitary Fibrous Tumor of The Deep Parotid GlandReyes Ivan García CuevasNo ratings yet
- Tumores en CuelloDocument6 pagesTumores en CuellokalixinNo ratings yet
- Angiomyolipoma of The Hard Palate: Clinical ReportDocument2 pagesAngiomyolipoma of The Hard Palate: Clinical ReportKezia Rachellea MustakimNo ratings yet
- Issn 2 Issn 2 Issn 2 ISSN 2073 073 073 073 - 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. SDocument7 pagesIssn 2 Issn 2 Issn 2 ISSN 2073 073 073 073 - 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. SAngga Witra NandaNo ratings yet
- A Case of Maxillary Sinus CarcinomaDocument3 pagesA Case of Maxillary Sinus CarcinomajasmniaNo ratings yet
- A Case of Giant Cell Tumor of The Rib With Magnetic Resonance ImagingDocument3 pagesA Case of Giant Cell Tumor of The Rib With Magnetic Resonance Imagingemilyleong86No ratings yet
- Management of Orbital Rhabdomyosarcoma: About 2 Cases and Literature ReviewDocument7 pagesManagement of Orbital Rhabdomyosarcoma: About 2 Cases and Literature ReviewIJAR JOURNALNo ratings yet
- Magnetic Resonance Imaging of MeningiomasDocument9 pagesMagnetic Resonance Imaging of MeningiomasElisabeth TikalakaNo ratings yet
- Surgical Neurology International: Unusual Growth Pattern of A MeningiomaDocument3 pagesSurgical Neurology International: Unusual Growth Pattern of A MeningiomaJamie NicholsNo ratings yet
- Anatomical Classification and Surgical Considerations: Primary Spinal Tumours An OverviewDocument5 pagesAnatomical Classification and Surgical Considerations: Primary Spinal Tumours An OverviewLyneth LacourtNo ratings yet
- Dr. Pranaya Kumar Panigrahi: PG StudentDocument104 pagesDr. Pranaya Kumar Panigrahi: PG Studentprabowoaji12No ratings yet
- Mekelle Universty College of Health Science Ayder Referral HospitalDocument68 pagesMekelle Universty College of Health Science Ayder Referral Hospitalprabowoaji12No ratings yet
- Pneumothorax in Neonate: M Hassan, M Begum, S M Z Haque, N Jahan, A Mannan, AwsrobDocument3 pagesPneumothorax in Neonate: M Hassan, M Begum, S M Z Haque, N Jahan, A Mannan, Awsrobprabowoaji12No ratings yet
- Techniques of Male Circumcision: ReviewDocument7 pagesTechniques of Male Circumcision: ReviewAnonymous XDbWQfD7No ratings yet
- Canmedaj01087 0002Document3 pagesCanmedaj01087 0002prabowoaji12No ratings yet
- Torsio Omentum 1Document5 pagesTorsio Omentum 1prabowoaji12No ratings yet
- SDK Bowel AnastomosisDocument43 pagesSDK Bowel Anastomosisprabowoaji12100% (1)
- Spinal Cord Injury Without Radiographic.23Document7 pagesSpinal Cord Injury Without Radiographic.23prabowoaji12No ratings yet
- Management of Valgus Knee With Irreducible Patella Disl With MCL RuptDocument8 pagesManagement of Valgus Knee With Irreducible Patella Disl With MCL Ruptprabowoaji12No ratings yet
- 3559-Article Text-11778-1-10-20200408Document7 pages3559-Article Text-11778-1-10-20200408prabowoaji12No ratings yet
- Primary Omental Torsion: A Case ReportDocument3 pagesPrimary Omental Torsion: A Case Reportprabowoaji12No ratings yet
- Snake BiteDocument206 pagesSnake BiteElviraThaherNo ratings yet
- A Rare Case of Acute Abdomen Secondary To Omental.283Document1 pageA Rare Case of Acute Abdomen Secondary To Omental.283prabowoaji12No ratings yet
- Jurnal Win JipDocument11 pagesJurnal Win Jipprabowoaji12No ratings yet
- SpleenDocument69 pagesSpleenprabowoaji12No ratings yet
- Pathophysiology of Pain in BiliodigestiDocument19 pagesPathophysiology of Pain in Biliodigestiprabowoaji12No ratings yet
- Pathophysiology of Pain in BiliodigestiDocument19 pagesPathophysiology of Pain in Biliodigestiprabowoaji12No ratings yet
- LysosomeDocument16 pagesLysosomeMd. Ismail HosenNo ratings yet
- AnatomyDocument55 pagesAnatomyLovely RamNo ratings yet
- Physiology of DeglutitionDocument23 pagesPhysiology of DeglutitionMkhaimar NihadNo ratings yet
- Cell ModificationDocument2 pagesCell ModificationArwen GarciaNo ratings yet
- Bone TumorsDocument1 pageBone Tumorsbfk777No ratings yet
- Chapter 9 Transport in Plants Lecture NotesDocument6 pagesChapter 9 Transport in Plants Lecture Notesann_hui_1No ratings yet
- CASE REPORT Dr. Arie Polim, SpOG FinalDocument50 pagesCASE REPORT Dr. Arie Polim, SpOG FinalpriskavkNo ratings yet
- Ultrasound in PregnancyDocument20 pagesUltrasound in PregnancyMuhammad AbeeshNo ratings yet
- Meanings of Houses in Vedic AstrologyDocument4 pagesMeanings of Houses in Vedic Astrologyshivashakti19746No ratings yet
- Grade 10 ReviewerDocument55 pagesGrade 10 ReviewerwkwjwjbsdNo ratings yet
- Microscopic Examination of UrineDocument93 pagesMicroscopic Examination of UrineJayniel Erys Molleno100% (1)
- Nervous System WorksheetDocument2 pagesNervous System WorksheetRoda Aranquez RabinoNo ratings yet
- Specific Immunity. FINALDocument29 pagesSpecific Immunity. FINALLUZVIMINDA GORDONo ratings yet
- Mbbs PhysioDocument27 pagesMbbs PhysioWwwanand111No ratings yet
- 10 Diseases - and - Immunity - Igcse Cie Biology - Ext Theory QP - UpdatedDocument9 pages10 Diseases - and - Immunity - Igcse Cie Biology - Ext Theory QP - Updatedmaryam2024igcseNo ratings yet
- Salt and Water BalanceDocument17 pagesSalt and Water BalancevonyNo ratings yet
- Seminar On Immunology-HypersensitivityDocument45 pagesSeminar On Immunology-HypersensitivitySalman KhanNo ratings yet
- Exchange Surfaces 2 QPDocument13 pagesExchange Surfaces 2 QPSaaleh AbanurNo ratings yet
- 9 SC TissueDocument8 pages9 SC Tissuevishesh1997No ratings yet
- Basic Science 2nd Term Jss2Document15 pagesBasic Science 2nd Term Jss2Adio Babatunde Abiodun Cabax100% (1)
- Pathophysiology of Alcoholic Liver DiseaseDocument4 pagesPathophysiology of Alcoholic Liver Diseaseshailendra tripathiNo ratings yet
- LP AnsDocument16 pagesLP AnsGayatri MishraNo ratings yet
- Cat SkeletonDocument10 pagesCat SkeletonJefferson TanNo ratings yet
- Histo CompilationDocument217 pagesHisto CompilationNico LokoNo ratings yet
- Lecture 3 Figure 1 - Motor TractsDocument9 pagesLecture 3 Figure 1 - Motor TractsSusanaGonzálezFernándezNo ratings yet
- Ch10 Kitabcd Class 8 MSBHSE Science NotesDocument14 pagesCh10 Kitabcd Class 8 MSBHSE Science NotesONE CLICK COMPUTERNo ratings yet
- VanPutte Seeleys Essentials 11e Chap06 PPT AccessibleDocument149 pagesVanPutte Seeleys Essentials 11e Chap06 PPT AccessibleCyrille Aira AndresaNo ratings yet