Professional Documents
Culture Documents
Chapter 5 The Synthesis of Materials and Imperfections: (Pyrolysis) or Absorption of UV Light (Photolysis)
Uploaded by
Gian BanaresOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 5 The Synthesis of Materials and Imperfections: (Pyrolysis) or Absorption of UV Light (Photolysis)
Uploaded by
Gian BanaresCopyright:
Available Formats
ChE 209 Materials Science and Engineering
Chapter 5 The Synthesis of Materials and Imperfections
34
Chapter 5 The Synthesis of Materials
And Imperfections
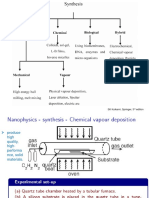
Gas to Solid Synthesis
1. Vapor deposition - involves the condensation of a vapor onto a cooled
substrate. Heating a solid powder (sublimation) or a liquid (evaporation)
achieves the vapor. The vapor deposition is one of the principal
techniques for preparing amorphous thin films of materials, ex. Silicon
and germanium.
2. Chemical vapor deposition - is a process whereby the vapor-phase
molecular species react, either homogenously in the gas phase or
heterogenously at the solid-gas interface at the surface of the substrate,
producing a film with a composition different from that of the starting
materials. The vapor molecules can be decomposed by means of heat
(pyrolysis) or absorption of UV light (photolysis).
Ex. Production of thin films of polycrystalline silicon by the thermal
decomposition of silane:
heat
SiH4 (g) Si (s) + H2 (g)
Another energy source that can be used to promote CVD is associated
with electrical plasma, a gas of ionized atoms and electrons. This is called
plasma-enhanced chemical vapor deposition. It is commonly used to
produce thin films of amorphous or polycrystalline silicon, depending on
the conditions, from silane.
3. Sputtering - is a process by which some of the atoms of an electrode,
usually a cathode, are ejected as a result of bombardment by heavy
positive ions. It is used to produce a clean surface or to deposit a uniform
film of metal on an object in an evacuated enclosure.
For the exclusive use of the UST Chemical Engineering students.
BMDuran
For the exclusive use of the UST Chemical Engineering Students
BMduran
ChE 209 Materials Science and Engineering
Chapter 5 The Synthesis of Materials and Imperfections
35
Atoms into gas state
Transport to substrate
Deposit on substrate
Liquid to Solid Synthesis
Bulk materials are often made starting from the liquid phase, either by
solidification of a melt to form single crystals when the cooling rate is
very low or to form non-crystalline materials when the cooling rate is
sufficiently fast that crystallization is prevented.
1. Crystal growth from the melt - Single-crystal materials (ultrapure)
can be produced in three methods: crystal is pulled out of the melt,
crystallization takes place in a crucible container and crystallization occurs
within a solid rod of material. The crystal-growth-technology is used in
electronics industry, where the requirements for material purity and
crystal perfection are extremely rigid.
2. Liquid quenching - involves super cooling of a liquid below its normal
freezing point. If a liquid is cooled quickly enough so that crystallization is
bypassed, a structurally disordered solid phase is formed.
3. Crystallization from solution - Preparation of crystals from solution
involves dissolving the material to be crystallized in a solvent and then
causing crystallization to occur, either by reducing the solubility of the
solute or by increasing the concentration of the solute in the solution by
removing the solvent. In both cases, a supersaturated solution is
produced from which crystals are formed. The process of crystallization
from a melt can be divided into two stages: nucleation and growth of
nuclei into crystals. In general, these are the steps in the solidification of
metals and alloys. The nucleation involves the clustering of atoms to form
nuclei. For a nucleus to be stable so that it can grow into a crystal, it must
reach a critical size. A cluster of atoms bonded together which is less than
the critical size is called an embryo, and one that is larger than the critical
size is called a nucleus. Because of their
For the exclusive use of the UST Chemical Engineering students.
BMDuran
For the exclusive use of the UST Chemical Engineering Students
BMduran
ChE 209 Materials Science and Engineering
Chapter 5 The Synthesis of Materials and Imperfections
36
instability, embryos are continuously being formed and redissolved in the
molten metal due to the agitation of the atoms. The stable nuclei then
grow in size by the transport or movement and rearrangement of atoms.
The Formation of Stable Nuclei in Liquid Metals
The two main mechanisms by which nucleation of solid particles in
liquid metal occurs are homogenous nucleation and heterogenous
nucleation
Homogenous Nucleation - It is the simplest case of nucleation.
Homogenous nucleation in a liquid melt occurs when the metal itself
provides the atom to form nuclei.
The two kinds of energy changes involved in homogenous nucleation are:
(1) the volume (or bulk) free energy (Gv) released by the liquid to solid
transformation and
(2) the specific free energy () required to form the new solid surfaces of
the solidified particles.
The total free energy change (GT) = (4/3)r3 (Gv) + 4r2
Heterogenous Nucleation - occurs in a liquid on the surfaces of its
container, insoluble impurities or other structural material which lowers
the critical free energy required forming a stable nucleus. In practice,
heterogenous nucleation is observed since the liquid is usually poured into
a mold that is always at a much lower temperature than the liquid and the
liquid always contains suspended particle impurities or nonmetallic
inclusions that provide surfaces on which nucleation will start.
Growth of Crystals in Liquid Metal and Formation of a Grain Structure
After stable nuclei have been formed in a solidifying metal, these nuclei grow
into crystals. When the solidification of the metal is finally completed, the
crystals join together in different orientations and form crystal boundaries at
which changes in orientation take place over a distance of a few atoms.
Solidified metal containing many crystals is said to be polycrystalline. The
crystals in the solidified metal are called grains and the surfaces between
them, grain boundaries.
Equiaxed grains - crystals grow equally in all directions
Columnar grains - long, thin coarse grains, which are created when a metal
solidifies relatively slowly in the presence of a steep temperature gradient.
For the exclusive use of the UST Chemical Engineering students.
BMDuran
For the exclusive use of the UST Chemical Engineering Students
BMduran
You might also like
- Pier Luigi IghinaDocument51 pagesPier Luigi Ighinagabriel100% (5)
- Self-Assembly of Nano- and Micro-structured Materials Using Colloidal EngineeringFrom EverandSelf-Assembly of Nano- and Micro-structured Materials Using Colloidal EngineeringNo ratings yet
- PreformulationDocument57 pagesPreformulationashpharma007100% (4)
- Solidification and Crystalline ImperfectionsDocument20 pagesSolidification and Crystalline ImperfectionsDavid IsaacNo ratings yet
- Dibaj Azhar 4Document6 pagesDibaj Azhar 4diba azharNo ratings yet
- MS Unit 5Document15 pagesMS Unit 5neha yarrapothuNo ratings yet
- English For MSE 9Document13 pagesEnglish For MSE 9mehdirashedipour0No ratings yet
- Nano Technology (Oe) - Unit Iii: Synthesis RoutesDocument104 pagesNano Technology (Oe) - Unit Iii: Synthesis RoutesDepartment of Chemical EngineeringNo ratings yet
- mt101 Part2Document46 pagesmt101 Part2Prateek 4-Yr B.Tech.: Metallurgical Engg., IIT(BHU)No ratings yet
- Methods of Producing Single Crystal: Author NoteDocument20 pagesMethods of Producing Single Crystal: Author NoteMohit SinhaNo ratings yet
- Metal JoiningDocument3 pagesMetal Joining006KAbhishek KumarNo ratings yet
- 3.1rev.4 Nature of MaterialsDocument41 pages3.1rev.4 Nature of MaterialsNguyễn Xuân NamNo ratings yet
- Crystal IzationDocument8 pagesCrystal Izationahmed.khallaf1962No ratings yet
- PT Unit 2Document5 pagesPT Unit 2C MohanNo ratings yet
- SK Kulkarni, Springer, 3 EditionDocument46 pagesSK Kulkarni, Springer, 3 EditionDarshit DesaiNo ratings yet
- Synthesis of NanomaterialsDocument55 pagesSynthesis of Nanomaterialsusama chaudhryNo ratings yet
- Course KGP003: Amorphous StructuresDocument25 pagesCourse KGP003: Amorphous StructuresrakukulappullyNo ratings yet
- PHY4207 - Chap 2 - Solidification PDFDocument49 pagesPHY4207 - Chap 2 - Solidification PDFshuhazllyNo ratings yet
- Classifications, Characterization and Applications of Metallic AlloysDocument35 pagesClassifications, Characterization and Applications of Metallic AlloysEidelsayedNo ratings yet
- Synthesis and Characterization of NanomaterialDocument157 pagesSynthesis and Characterization of NanomaterialShriyansh JainNo ratings yet
- Synth 1 PDFDocument7 pagesSynth 1 PDFSelva BabuNo ratings yet
- Synth 1Document7 pagesSynth 1Selva BabuNo ratings yet
- Solidification of AlloysDocument18 pagesSolidification of AlloysKONDRAPALLY ANJANEYULUNo ratings yet
- Composite Fabrication ProcessDocument29 pagesComposite Fabrication ProcessSaiprajithNo ratings yet
- Module 3Document17 pagesModule 3sathwikkumasamaNo ratings yet
- Lecture 1 (Part 2)Document24 pagesLecture 1 (Part 2)Lance BienNo ratings yet
- LECTURE 15-1: Synthesis and Preparation of Nanomaterials (Crystalline and Thinfilm)Document30 pagesLECTURE 15-1: Synthesis and Preparation of Nanomaterials (Crystalline and Thinfilm)Sk. Laila AyeshaNo ratings yet
- New Unit - IIIDocument44 pagesNew Unit - IIIAdi KothaNo ratings yet
- Unit 2-Chapter 4 - Solidification and Phase DiagramsDocument105 pagesUnit 2-Chapter 4 - Solidification and Phase Diagramssainath reddy kesam reddyNo ratings yet
- Presence of A Translucent Stage of Super Cooled Fluid or A Supersaturated DissolvableDocument2 pagesPresence of A Translucent Stage of Super Cooled Fluid or A Supersaturated DissolvableHas SimNo ratings yet
- Metal Casting & Welding 15Me35ADocument20 pagesMetal Casting & Welding 15Me35A01061975No ratings yet
- CH 6-Cooling and Solidification of CastingDocument33 pagesCH 6-Cooling and Solidification of CastingGosaye Desalegn100% (1)
- Glossary Solidification Part 1Document2 pagesGlossary Solidification Part 1ayman aydanNo ratings yet
- Unesco - Eolss Sample Chapters: Processing From The Liquid StateDocument5 pagesUnesco - Eolss Sample Chapters: Processing From The Liquid StateER NurNo ratings yet
- 2 SolidificacionDocument63 pages2 SolidificacionAndrea Espinosa OrtegaNo ratings yet
- Nano Unit 1 NotesDocument17 pagesNano Unit 1 NotesajitsssNo ratings yet
- CHAPTER 4:solid Solution Equilibrium Phase Diagram Name:Muhammad Akmal Afiq Bin Amran MATRIK:11DKM19F1028Document20 pagesCHAPTER 4:solid Solution Equilibrium Phase Diagram Name:Muhammad Akmal Afiq Bin Amran MATRIK:11DKM19F1028Muhd AriffNo ratings yet
- Amorphous Lecture PDFDocument25 pagesAmorphous Lecture PDFBá Văn TôNo ratings yet
- Chapter9 EscabarteDocument30 pagesChapter9 EscabarteDarwin CruzNo ratings yet
- Microfabrication J Thin Film Method For Micro Fuel CellDocument26 pagesMicrofabrication J Thin Film Method For Micro Fuel CellSajid BabuNo ratings yet
- 6 Solid SolutionsDocument25 pages6 Solid SolutionsKiran RajNo ratings yet
- Solid-State Chemistry, (Also Called Materials Chemistry) Is The Study ofDocument11 pagesSolid-State Chemistry, (Also Called Materials Chemistry) Is The Study ofs sunil k kumarNo ratings yet
- Thin FilmDocument42 pagesThin FilmBhagyashree PaniNo ratings yet
- Crystal Growth and Wafer PreparationDocument18 pagesCrystal Growth and Wafer PreparationraghudatheshNo ratings yet
- Eee Module 1Document75 pagesEee Module 1Anitha BRNo ratings yet
- Solidification: A. Crystallization and The Development of Cast StructureDocument5 pagesSolidification: A. Crystallization and The Development of Cast StructureAsif AhmedNo ratings yet
- Semiconductor Fabrication Lecture NotesDocument11 pagesSemiconductor Fabrication Lecture NotesJoanna Fabricante100% (1)
- Solidification and Phase DiagramsDocument60 pagesSolidification and Phase DiagramsnattydreadfathelahNo ratings yet
- Material Science - CompressedDocument82 pagesMaterial Science - CompressedMatthew SmithNo ratings yet
- On SolidificationDocument11 pagesOn Solidificationamalendu_biswas_1No ratings yet
- Casting Slides 56-66Document11 pagesCasting Slides 56-66Asjad khanNo ratings yet
- (Chemistry) Sol Gel and Organic ReactionsDocument36 pages(Chemistry) Sol Gel and Organic ReactionsAshutosh TripathyNo ratings yet
- Modern Materials Prepared With The Use of Levitation Melting Method in The Magnetic FieldsDocument5 pagesModern Materials Prepared With The Use of Levitation Melting Method in The Magnetic FieldsArdu StuffNo ratings yet
- Metal Casting & Welding 15Me35ADocument20 pagesMetal Casting & Welding 15Me35ASHEKHARAPPA MALLURNo ratings yet
- Assignment in Metallic Glasses PDFDocument18 pagesAssignment in Metallic Glasses PDFVivek HanchateNo ratings yet
- Chapter 4 Solid Solution Equilibrium Phase Diagram PDFDocument41 pagesChapter 4 Solid Solution Equilibrium Phase Diagram PDFSergio Syamil100% (2)
- 1D Semiconductor and AuNPsDocument54 pages1D Semiconductor and AuNPsyared mulgetaNo ratings yet
- MT 611 Physical MetallurgyDocument85 pagesMT 611 Physical MetallurgyRajarajan KrishnamoorthyNo ratings yet
- Unit 3: Aircraft Material ScienceDocument33 pagesUnit 3: Aircraft Material Scienceprathik sNo ratings yet
- MODULEDocument82 pagesMODULENithin GopalNo ratings yet
- Understanding Our Environment HandoutDocument9 pagesUnderstanding Our Environment HandoutGian BanaresNo ratings yet
- Ozone Layer Depletion: Solar RadiationDocument9 pagesOzone Layer Depletion: Solar RadiationGian BanaresNo ratings yet
- Science of The Environment: The Earth's Cross SectionDocument7 pagesScience of The Environment: The Earth's Cross SectionGian BanaresNo ratings yet
- 2 Ecosystems Biomes-2011Document14 pages2 Ecosystems Biomes-2011Gian BanaresNo ratings yet
- ChE 311 Problem Set With Answer KeyDocument5 pagesChE 311 Problem Set With Answer KeyGian BanaresNo ratings yet
- Fragrances and Essential OilsDocument32 pagesFragrances and Essential OilsGian BanaresNo ratings yet
- Group 4 - Fermentation Industry (Handouts)Document6 pagesGroup 4 - Fermentation Industry (Handouts)Gian BanaresNo ratings yet
- Science of The Environment: Earth FactsDocument3 pagesScience of The Environment: Earth FactsGian BanaresNo ratings yet
- Industrial Chem Post Lab 4 - Physico-Chemical Properties of Soaps and DetergentsDocument4 pagesIndustrial Chem Post Lab 4 - Physico-Chemical Properties of Soaps and DetergentsGian BanaresNo ratings yet
- Biodiversity Notes From WWFDocument6 pagesBiodiversity Notes From WWFGian BanaresNo ratings yet
- 1 AtmoSPHERESDocument3 pages1 AtmoSPHERESGian BanaresNo ratings yet
- Chapter 4Document38 pagesChapter 4Gian BanaresNo ratings yet
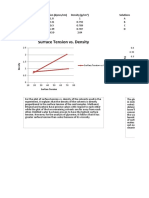
- Surface Tension vs. Density Surface Tension vs. Detergent ConcentrationDocument2 pagesSurface Tension vs. Density Surface Tension vs. Detergent ConcentrationGian BanaresNo ratings yet
- Physical Chemistry Post Lab - Experiment 3 Surface TensionDocument8 pagesPhysical Chemistry Post Lab - Experiment 3 Surface TensionGian BanaresNo ratings yet
- Ceramics: Automobiles (Sparkplugs and Ceramic Engine Parts Found in Racecars), and Phone Lines. TheyDocument8 pagesCeramics: Automobiles (Sparkplugs and Ceramic Engine Parts Found in Racecars), and Phone Lines. TheyGian BanaresNo ratings yet
- Biomaterial Classifications of Biomaterials Based On CompositionDocument2 pagesBiomaterial Classifications of Biomaterials Based On CompositionGian BanaresNo ratings yet
- Chapter 3: Fundamentals of Crystallography: Issues To Address..Document12 pagesChapter 3: Fundamentals of Crystallography: Issues To Address..Gian BanaresNo ratings yet
- Chapters 9-10-11Document73 pagesChapters 9-10-11Gian BanaresNo ratings yet
- Heat Treatment of MetalsDocument7 pagesHeat Treatment of MetalsGian BanaresNo ratings yet
- Mechanical Properties of Metals: Issues To Address..Document30 pagesMechanical Properties of Metals: Issues To Address..Gian BanaresNo ratings yet
- Group 5Document32 pagesGroup 5Gian BanaresNo ratings yet
- MSE CompositesDocument11 pagesMSE CompositesGian BanaresNo ratings yet
- Chapter 5Document131 pagesChapter 5Gian BanaresNo ratings yet
- MSE - METALS Report (Draft)Document6 pagesMSE - METALS Report (Draft)Gian BanaresNo ratings yet
- Chapter 2 - 1Document20 pagesChapter 2 - 1Gian BanaresNo ratings yet
- Organic Chemistry Chapter 5 - StereochemistryDocument89 pagesOrganic Chemistry Chapter 5 - StereochemistryGian BanaresNo ratings yet
- Chapter 1 - 1Document18 pagesChapter 1 - 1Gian BanaresNo ratings yet
- Organic Chemistry Chapter 3 - An Introduction To Organic Reactions and Their MechanismsDocument81 pagesOrganic Chemistry Chapter 3 - An Introduction To Organic Reactions and Their MechanismsGian BanaresNo ratings yet
- Condensed Phases - Liquids and SolidsDocument7 pagesCondensed Phases - Liquids and SolidspanocomNo ratings yet
- A Genetic Model For Na-Carbonate Mineral Precipitation in The MioceneDocument13 pagesA Genetic Model For Na-Carbonate Mineral Precipitation in The MioceneYiğitcan AkyüzNo ratings yet
- Chm361-Chapter 3 SolidDocument60 pagesChm361-Chapter 3 Solidfatin harrisNo ratings yet
- R17 Mechatronics Syllabus PDFDocument112 pagesR17 Mechatronics Syllabus PDFScientist SakthivelNo ratings yet
- General Chemistry 2Document11 pagesGeneral Chemistry 2Fayza Jalil BaladingNo ratings yet
- Nomenclature of The Forms of Crystalline and Non-Crystalline SilicaDocument9 pagesNomenclature of The Forms of Crystalline and Non-Crystalline SilicasudiptodattaNo ratings yet
- Chapter 1Document36 pagesChapter 1Umesh ChandraNo ratings yet
- GLY 206 NOTE 3 - Crystal StructureDocument20 pagesGLY 206 NOTE 3 - Crystal StructureOdebunmi PaulNo ratings yet
- XLPE Polyethylene Lifetime Detection PDFDocument120 pagesXLPE Polyethylene Lifetime Detection PDFconqiuNo ratings yet
- The Britannica Guide To Matter PDFDocument294 pagesThe Britannica Guide To Matter PDFElizebethNo ratings yet
- Minerals - John P. RaffertyDocument358 pagesMinerals - John P. RaffertyBeatriz Camara100% (14)
- MaterialogyDocument181 pagesMaterialogyrajraj3550No ratings yet
- Phases of MatterDocument2 pagesPhases of MatterDrixie Mae G. LaraziNo ratings yet
- Crystal HandoutsDocument23 pagesCrystal HandoutsArup DasNo ratings yet
- Calcite, Dolomite, AragoniteDocument11 pagesCalcite, Dolomite, AragoniteMeitri Wulandari Kohar100% (1)
- Physical and Chemical Stability and Excipient CompatibilityDocument12 pagesPhysical and Chemical Stability and Excipient CompatibilityRebecca ChenNo ratings yet
- Network Former & ModifierDocument28 pagesNetwork Former & ModifierMaharani PutriNo ratings yet
- Crystallography and Structure - Ch3F10-2Document66 pagesCrystallography and Structure - Ch3F10-2Syeda Fatima FarzamNo ratings yet
- Lecture 1 Semi ConducteurDocument31 pagesLecture 1 Semi Conducteurnour1960No ratings yet
- Structures and Properties of Substances: Learning GoalDocument44 pagesStructures and Properties of Substances: Learning GoalRyanNo ratings yet
- Course Gem IdentificationDocument79 pagesCourse Gem IdentificationAnonymous IcptMgwZlNo ratings yet
- Relation Between Coal and Fly Ash Mineralogy Based On Quantitative X Ray DiffractionDocument14 pagesRelation Between Coal and Fly Ash Mineralogy Based On Quantitative X Ray DiffractionDrsttNo ratings yet
- Defects Crystals: Never Give Up Coming Soon 32 1Document15 pagesDefects Crystals: Never Give Up Coming Soon 32 1fita komalaNo ratings yet
- Tech Tip 7 - Epoxy CrystallizationDocument2 pagesTech Tip 7 - Epoxy CrystallizationflavioferiNo ratings yet
- 1st Year NEP AY 2023-24Document10 pages1st Year NEP AY 2023-24Dhruv kashyapNo ratings yet
- The PAH WorldDocument14 pagesThe PAH WorldCarlos Guillermo Reales GonzalezNo ratings yet
- Properties of Palm Oil MargarineDocument7 pagesProperties of Palm Oil Margarinemcalidonio5656No ratings yet
- Concretion Morphology, Classification and GenesisDocument34 pagesConcretion Morphology, Classification and GenesisJohan NikoNo ratings yet