Professional Documents
Culture Documents
Dalton's Law of Partial Pressure: Mathematically
Uploaded by
Nabila Azzahra SalsabilaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Dalton's Law of Partial Pressure: Mathematically
Uploaded by
Nabila Azzahra SalsabilaCopyright:
Available Formats
Lec. 3 Dalton’s Law & Real gas …………… …………………………………. Haider R.
Saud
Dalton’s Law of Partial Pressure
The relation between the pressures of the mixture of non-reacting gases
enclosed in a vessel to their individual pressure is described in the law.
The law was given by John Dalton in 1807.
At constant temperature, the pressure exerted by a mixture of two or more
non-reacting gases enclosed in a definite volume, is equal to the sum of the
individual pressures which each gas would exert if present alone in the
same volume.
The individual pressures of gases are known as partial pressures.
Mathematically
If P is the total pressure of the mixture of non-reacting gases at temperature
T and volume V, and P1, P2, P3 …. represent the partial pressures of the
gases, then
P = P1 + P2 + P3+ ……… (T, V are constant)
Partial Pressure in terms of Mole Fraction
Mole fraction (X) defines the amount of a substance in a mixture as a
fraction of total amount of all substances.
If n =1 moles of any substance is present in n moles of the mixture, then
mole fraction of the substance,
X1 = n1 / n
X1 = P1 / P
P1 = X1 × P
P = P1 + P2 + P3 + ………. + Pi
n = n1 + n2 + n3 + ………. + ni
1 University of Al Muthana, college of science, chemistry department
Lec. 3 Dalton’s Law & Real gas …………… …………………………………. Haider R. Saud
Question:
A 2.5 litre flask contains 0.25 mole each of sulphur dioxide and nitrogen gas at
27°C. Calculate the partial pressure exerted by each gas and also the total
pressure.
Solution:
Partial pressure of SO2
PSO2 = nRT / V = 0.25 × 8.314 × 300 / 2.5 × 10–3 = 2.49 × 105 Nm–2 = 2.49 ×
105 Pa
Similarly PN2 = 2.49 × 105 Pa
Following Dalton’s Law
PTotal = PN2 + PSO2 = 2.49 × 105 Pa + 2.49 × 105 Pa = 4.98 × 105 Pa
H.W) A vessel of volume 22.4 dm3 contains 1.5 mol H2 and 2.5 mol N2 at
273.1 K. Calculate (a) the mole fractions of each component, (b) their partial
pressures, and (c) their total pressure.
Real gas
A gas which obeys the gas laws and the gas equation PV = nRT strictly at all
temperatures and pressures is said to be an ideal gas. The molecules of ideal
gases are assumed to be volume less points with no attractive forces between
one another. But no real gas strictly obeys the gas equation at all temperatures
and pressures. Deviations from ideal behaviour are observed particularly at
high pressures or low temperatures.
2 University of Al Muthana, college of science, chemistry department
Lec. 3 Dalton’s Law & Real gas …………… …………………………………. Haider R. Saud
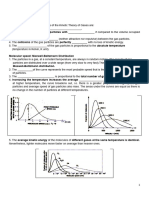
The deviation from ideal behaviour is expressed by introducing a factor Z
known as compressibility factor in the ideal gas equation. Z may be
expressed as Z = PV / nRT
In case of ideal gas, PV = nRT ∴ Z = 1
In case of real gas, PV ≠ nRt ∴ Z ≠ 1
Thus in case of real gases Z can be < 1 or > 1
When Z < 1, it is a negative deviation. It shows that the gas is more
compressible than expected from ideal behaviour.
When Z > 1, it is a positive deviation. It shows that the gas is less
compressible than expected from ideal behaviour.
Question
1 mole of SO2 occupies a volume of 350 ml at 300K and 50 atm pressure.
Calculate the compressibility factor of the gas.
Solution:
P = 50 atm
V = 350 ml = 0.350 litre
n = 1 mole
T = 300L Z = PV / nRT
∴ Z = 50 × 0.350 / 1 × 0.082 × 300 = 0.711
Thus SO2 is more compressible than expected from ideal behaviour.
3 University of Al Muthana, college of science, chemistry department
Lec. 3 Dalton’s Law & Real gas …………… …………………………………. Haider R. Saud
Van der Waals’ Equation of State for a Real Gas
This equation can be derived by considering a real gas and 'converting ' it to
an ideal gas.
Volume Correction
We know that for an ideal gas P´V = nRT. Now in a real gas the molecular
volume cannot be ignored and therefore let us assume that 'b' is the volume
excluded (out of the volume of container) for the moving gas molecules per
mole of a gas. Therefore due to n moles of a gas the volume excluded would
be nb. \ a real gas in a container of volume V has only available volume of (V
- nb) and this can be thought of as an ideal gas in container of volume (V -
nb).
Hence, Ideal volume
Vi = V – nb …..........(i)
Pressure Correction
It can be seen that the pressure the real gas exerts would be less than the
pressure an ideal gas would have exerted. The real gas experiences attractions
by its molecules in the reverse direction. Therefore if a real gas exerts a
pressure P, then an ideal gas would exert a pressure equal to P + p (p is the
pressure lost by the gas molecules due to attractions).
Therefore p ∝ n/v (concentration of molecules which are hitting the
container's wall)
P ∝ n/v (concentration of molecules which are attracting these
molecules) p ∝ n2/v2
P = an2/v2 where a is the constant of proportionality which depends on the
nature of gas.
4 University of Al Muthana, college of science, chemistry department
Lec. 3 Dalton’s Law & Real gas …………… …………………………………. Haider R. Saud
Pi = (P + an2 / V2) …...........(ii)
Here,
a = A constant whose value depends upon the nature of the gas
Substituting the values of ideal volume and ideal pressure in ideal gas equation
i.e. pV=nRT, the modified equation is obtained as
nRT n 2a
or P 2
V nb V
The constants a & b
Vander Waals constant for attraction (a) and volume (b) are characteristic for a
given gas. Some salient feature of a & b are:
For a given gas Vander Waal’s constant of attraction ‘a’ is always greater than
Vander Waals constant of volume (b).
The units of a = litre2 atm mole–2 & that of b = litre mole –1
Some Other Important Definitions
Critical Temperature (Tc)
It (Tc) is the maximum temperature at which a gas can be liquefied i.e. the
temperature above which a gas can’t exist as liquid.
Tc = 8a / 27Rb
Critical Pressure (Pc)
It is the minimum pressure required to cause liquefaction at Tc
Pc = a/27b2
5 University of Al Muthana, college of science, chemistry department
Lec. 3 Dalton’s Law & Real gas …………… …………………………………. Haider R. Saud
Critical Volume
It is the volume occupied by one mol of a gas at Tc and Pc
Vc = 3b
Q) find the pressure of 2.00 moles of carbon dioxide gas at 298 K in a 5.00 L
container. The van der Waals constants for carbon dioxide are: a = 3.592 L2.
atm/mol2 and b = 0.04267 L/mol.
nRT n 2a
P 2
V nb V
(2.00 mol)(0.0821 L atm)(298 K) (2.00 mol)2(3.592 L2 atm)
P 9.38 atm
5.00 L (2.00 mol)mol K(0.04267 L)
2
(5.00 L) mol2
Compare this to the pressure calculated using the ideal gas law:
nRT
P
V
(2.00 mol)(0.0821 L atm)(298 K)
P 9.77 atm
(5.00 L)mol K
Q) The critical constants of ethane are pC = 48.20 atm, Vc= 148 cm and Tc =
305.4 K. Calculate the van der Waals parameters of the gas (a, b)
H.w) The critical constants of methane are pc = 45.6 atm, Vc = 98.7 cm3
, and Tc= 190.6 K. Calculate the van der Waals parameters of the gas.
6 University of Al Muthana, college of science, chemistry department
You might also like
- Audels Engineers and Machinists Guide Vol1 PDFDocument486 pagesAudels Engineers and Machinists Guide Vol1 PDFloosenutNo ratings yet
- Study Guide Gas LawsDocument3 pagesStudy Guide Gas LawsAdamNo ratings yet
- Course Structure - Agricultural EngineeringDocument15 pagesCourse Structure - Agricultural EngineeringKanda Wisdom75% (4)
- CHM 121 - Lecture Note 7 - Kinetic Theory of Gases, Gas Laws, EquationsDocument35 pagesCHM 121 - Lecture Note 7 - Kinetic Theory of Gases, Gas Laws, Equationssomide kayodeNo ratings yet
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsFrom EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsRating: 5 out of 5 stars5/5 (1)
- Disha Publication Previous Years Problems On Thermodynamics For NEET. CB1198675309Document11 pagesDisha Publication Previous Years Problems On Thermodynamics For NEET. CB1198675309Study UT educationNo ratings yet
- PHY 103 Equations of StateDocument37 pagesPHY 103 Equations of Statebishal alamNo ratings yet
- States of Matter Course OutlineDocument40 pagesStates of Matter Course OutlinedevoydouglasNo ratings yet
- Pneumatic Leak Testing Procedure for Reinforcement PadsDocument4 pagesPneumatic Leak Testing Procedure for Reinforcement PadsHansel Francis100% (5)
- The 8 Gas Laws ExplainedDocument55 pagesThe 8 Gas Laws ExplainedRyan RamlawiNo ratings yet
- Conversion of Gas Flow From m3 To Nm3Document2 pagesConversion of Gas Flow From m3 To Nm3vvijaybhan100% (1)
- Gas Density CalculationDocument10 pagesGas Density CalculationKhurshid AhmadNo ratings yet
- Gas Laws (Chem)Document27 pagesGas Laws (Chem)EncounteriGH100% (3)
- ChE ReviewerDocument27 pagesChE ReviewerJohn Paul Rodriguez100% (2)
- Organic Agricultural Chemistry (1916) - J Chamberlain PDFDocument356 pagesOrganic Agricultural Chemistry (1916) - J Chamberlain PDFbabithy100% (1)
- Gases and Their Properties: Exercises, Examples, and BOLD Numbered ProblemsDocument106 pagesGases and Their Properties: Exercises, Examples, and BOLD Numbered ProblemsMia YukimuraNo ratings yet
- ChapterII - GasesDocument40 pagesChapterII - Gasesjumanahelmy12No ratings yet
- CH 10 Gases StudentDocument48 pagesCH 10 Gases StudentTrọng NguyễnNo ratings yet
- Lec 3Document14 pagesLec 3Not EmeraruduNo ratings yet
- Chemistry Form 6 Sem 1 04Document64 pagesChemistry Form 6 Sem 1 04Ng Swee Loong Steven100% (6)
- 5.0 States of MatterDocument106 pages5.0 States of MatterTasya KassimNo ratings yet
- Gaseous States Chapter: Gas Laws, Ideal Gas Law, Kinetic Molecular TheoryDocument78 pagesGaseous States Chapter: Gas Laws, Ideal Gas Law, Kinetic Molecular TheoryHiep NguyenNo ratings yet
- Gaseous State (Ideal Gases) Exercise With Sol.Document25 pagesGaseous State (Ideal Gases) Exercise With Sol.mikcNo ratings yet
- Unit 1Document26 pagesUnit 1firehywotNo ratings yet
- Gaseous StateDocument14 pagesGaseous StatemayankNo ratings yet
- Chapter 4 States of Matter 2021Document24 pagesChapter 4 States of Matter 2021suh mey chongNo ratings yet
- Gas Laws: CHEM140 February 2, 2005Document33 pagesGas Laws: CHEM140 February 2, 2005elokflhNo ratings yet
- Chapter10 NotesDocument33 pagesChapter10 NotesHeather WrightNo ratings yet
- Fundamentals of Gas LawsDocument9 pagesFundamentals of Gas LawsMangojuice MbeleNo ratings yet
- Introduction To Physical Chemistry: 2. Real Gases and Van Der Waals EquationsDocument13 pagesIntroduction To Physical Chemistry: 2. Real Gases and Van Der Waals EquationsDery RachmandaniNo ratings yet
- Chem 181 Chemistry of GasesDocument15 pagesChem 181 Chemistry of GasesJoey PooleNo ratings yet
- Chapter 5Document12 pagesChapter 5DavidVizcaínoNo ratings yet
- Van Der Waal's EquationDocument7 pagesVan Der Waal's EquationRosse KNo ratings yet
- 6_2021_09_19!01_48_26_PMDocument11 pages6_2021_09_19!01_48_26_PMliennev02No ratings yet
- Chapter 5 Gases PDFDocument49 pagesChapter 5 Gases PDFAbou WalidNo ratings yet
- Chapter - 5 State of MatterDocument5 pagesChapter - 5 State of MatterManan TyagiNo ratings yet
- R ConstantDocument14 pagesR ConstantMika LavadoNo ratings yet
- 5.1 Pressure: Chapter 5: GasesDocument4 pages5.1 Pressure: Chapter 5: GasesSam ChungNo ratings yet
- CHM 101 Lecture Note-Gas LawsDocument11 pagesCHM 101 Lecture Note-Gas LawsMichael DanielsNo ratings yet
- Gas Laws:: P V K VDocument18 pagesGas Laws:: P V K VFarah Zu'biNo ratings yet
- Topic 05 - States of Matter - TutorsDocument17 pagesTopic 05 - States of Matter - TutorsTran Nhat ThangNo ratings yet
- Chapter 5 CHEM110Document59 pagesChapter 5 CHEM110gracetetu102No ratings yet
- 4.1 Ideal GasesDocument22 pages4.1 Ideal GasesAnonymous o97HYLpe0No ratings yet
- Gases 2.1 Pressure and Temperature Pressure: Liquide HG Liquide HGDocument11 pagesGases 2.1 Pressure and Temperature Pressure: Liquide HG Liquide HGapi-26639719No ratings yet
- SC RE Chap5-GasesDocument49 pagesSC RE Chap5-Gasesweldsv0% (1)
- Silo - Tips - Chapter 5 The Gaseous StateDocument18 pagesSilo - Tips - Chapter 5 The Gaseous StateJerich Ivan PaalisboNo ratings yet
- Properties of Mixtures Mixture of Perfect GasesDocument7 pagesProperties of Mixtures Mixture of Perfect GasesAudu SanusiNo ratings yet
- Here are the answers:1) a) Quality = 0.6 b) Superheated, T = 100°C2) 5.3 MPa 3) a) Yes b) Yes c) No4) 3.98 MPaDocument18 pagesHere are the answers:1) a) Quality = 0.6 b) Superheated, T = 100°C2) 5.3 MPa 3) a) Yes b) Yes c) No4) 3.98 MPaAditya SuryawanshiNo ratings yet
- Gas Laws LecDocument43 pagesGas Laws LecJune Francis AngNo ratings yet
- Gases and Other Properties: Lesson 5Document7 pagesGases and Other Properties: Lesson 5lucifer angelNo ratings yet
- Thermal Properties of Matter Sample ProblemsDocument4 pagesThermal Properties of Matter Sample ProblemsEdogawaNo ratings yet
- Chapter 10 Jan13Document104 pagesChapter 10 Jan13kumuthaNo ratings yet
- Chapter 10 Sept13Document57 pagesChapter 10 Sept13chandro57No ratings yet
- Gas PropertiesDocument54 pagesGas PropertiesAli AhmedNo ratings yet
- BSG 104 Gas LawsDocument35 pagesBSG 104 Gas LawsCJ DRBNo ratings yet
- Gases 2.1 Pressure and Temperature Pressure: Liquide HG Liquide HGDocument8 pagesGases 2.1 Pressure and Temperature Pressure: Liquide HG Liquide HGapi-26639719No ratings yet
- Week 3 PPT AD CHEMDocument8 pagesWeek 3 PPT AD CHEMSophia Ysabelle EstradaNo ratings yet
- Gas Laws Ws PDFDocument6 pagesGas Laws Ws PDFJulia Franchesca BorromeoNo ratings yet
- CHM131 - Chapter 6 - The Gaseous StateDocument37 pagesCHM131 - Chapter 6 - The Gaseous StateNotes NotesNo ratings yet
- Department of ChemistryDocument15 pagesDepartment of ChemistryWorcPrimerNo ratings yet
- GAS PROPERTIES: IDEAL VS REALDocument9 pagesGAS PROPERTIES: IDEAL VS REALReza Gustarani DaneswariNo ratings yet
- Chapter 5 GasesDocument8 pagesChapter 5 GasessamNo ratings yet
- 8th 10th Day GasesDocument78 pages8th 10th Day GasesIsabel Velan ViernesNo ratings yet
- Chapter 1Document30 pagesChapter 1Siti Hajar Mohd PodziNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Recommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsFrom EverandRecommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsE. F. G. HeringtonNo ratings yet
- Fiitjee Rit 2Document11 pagesFiitjee Rit 2Baljeet SinghNo ratings yet
- Zadaci Fizika - EngDocument13 pagesZadaci Fizika - EngAljoša GraovacNo ratings yet
- Chem 156 4th Departmental Exam Advanced ProblemsDocument1 pageChem 156 4th Departmental Exam Advanced Problemsapi-3856754No ratings yet
- Pipe Flow & Pressure Drop CalculatorsDocument7 pagesPipe Flow & Pressure Drop CalculatorsdasubhaiNo ratings yet
- Applications in Aviation HaskelDocument4 pagesApplications in Aviation HaskelMouhaNo ratings yet
- 6 Aug KVPY SA Chemistry 2Document12 pages6 Aug KVPY SA Chemistry 2riddhiNo ratings yet
- Materials Chemistry and Physics: High Temperature Stability of Surfactant Capped Cofe O NanoparticlesDocument7 pagesMaterials Chemistry and Physics: High Temperature Stability of Surfactant Capped Cofe O NanoparticlesZuhrotul AiniNo ratings yet
- Thermo AutosavedDocument140 pagesThermo AutosavedMae noreen TobiasNo ratings yet
- TE-1 Question BankDocument7 pagesTE-1 Question BankMANJUNATH V BNo ratings yet
- Phase Changes GuideDocument33 pagesPhase Changes GuideRonajane KitoyNo ratings yet
- Chemistry Question Bank For JEE Advance Part 1Document55 pagesChemistry Question Bank For JEE Advance Part 1gfffdssseNo ratings yet
- Iit Jee Main SyllabusDocument25 pagesIit Jee Main SyllabusIITIAN SANJEEV[IITK]No ratings yet
- Introduction To Fuels: Gaseous FuelDocument2 pagesIntroduction To Fuels: Gaseous Fuelkcp1986No ratings yet
- MECHANICAL ENGINEERING 2019 Scheme S4 Syllabus Ktustudents - inDocument91 pagesMECHANICAL ENGINEERING 2019 Scheme S4 Syllabus Ktustudents - inashnbNo ratings yet
- Jest 2017Document15 pagesJest 2017r prathapNo ratings yet
- F5 Chapter 1 Rate of ReactionDocument18 pagesF5 Chapter 1 Rate of ReactionSiti Aishah ZolkanainNo ratings yet
- Chemical Thermodynamics Quick RevisionDocument17 pagesChemical Thermodynamics Quick RevisionGaurav ChaudharyNo ratings yet
- How To Produce Metanol (Ebook) PDFDocument210 pagesHow To Produce Metanol (Ebook) PDFelfainsyahNo ratings yet
- Effect of Cycling Operations On An Adsorbed Natural Gas StorageDocument9 pagesEffect of Cycling Operations On An Adsorbed Natural Gas StorageMartín KunuschNo ratings yet
- MAT 435 Chapter 3: Functions of Two and Three Variables: 1 y X 3) Y, X (FDocument8 pagesMAT 435 Chapter 3: Functions of Two and Three Variables: 1 y X 3) Y, X (FlancaugilaNo ratings yet
- Cook Static PakDocument2 pagesCook Static PakAlfred LamNo ratings yet
- MathDocument43 pagesMathElijah John De leonNo ratings yet
- 105 Converting Coarse Bubble Aeration To Fine BubbleDocument3 pages105 Converting Coarse Bubble Aeration To Fine BubbleLTE002No ratings yet