Professional Documents
Culture Documents
Sympathetic Nervous Tone Limits The Development of Myeloid-Derived Suppressor Cells
Uploaded by
Sol LakosOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Sympathetic Nervous Tone Limits The Development of Myeloid-Derived Suppressor Cells
Uploaded by
Sol LakosCopyright:
Available Formats
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
MYELOID CELLS Copyright © 2020
The Authors, some
Sympathetic nervous tone limits the development rights reserved;
exclusive licensee
of myeloid-derived suppressor cells American Association
for the Advancement
of Science. No claim
James T. Nevin1*, Marmar Moussa2, William L. Corwin1, Ion I. Mandoiu2, Pramod K. Srivastava1* to original U.S.
Government Works
Sympathetic nerves that innervate lymphoid organs regulate immune development and function by releasing
norepinephrine that is sensed by immune cells via their expression of adrenergic receptors. Here, we demonstrate
that ablation of sympathetic nervous system (SNS) signaling suppresses tumor immunity, and we dissect the mech-
anism of such immune suppression. We report that disruption of the SNS in mice removes a critical -adrenergic
signal required for maturation of myeloid cells in normal and tumor-bearing mice. In tumor-bearing mice, disrup-
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
tion of the -adrenergic signal leads to the accumulation of immature myeloid-derived suppressor cells (MDSCs)
that suppress tumor immunity and promote tumor growth. Furthermore, we show that these SNS-responsive
MDSCs drive expansion of regulatory T cells via secretion of the alarmin heterodimer S100A8/A9, thereby com-
pounding their immunosuppressive activity. Our results describe a regulatory framework in which sympathetic
tone controls the development of innate and adaptive immune cells and influences their activity in health and
disease.
INTRODUCTION cytotoxic T cell response and alteration of the T helper and myeloid
The sympathetic nervous system (SNS) has a complex, bidirectional compartments toward a more suppressive phenotype (17–19). Ac-
relationship with the immune system (1, 2). Various immune cell cumulation of MDSCs in tumors is correlated with poor outcomes
types, including myeloid cells and T cells, have the ability to respond in cancer (20, 21) and is a major barrier to developing effective can-
to SNS signaling via expression of adrenergic receptors (ARs) on cer immunotherapy; thus, understanding how they develop and are
their surface (3). Perturbations of the SNS have been demonstrated regulated is critically important.
to affect cellular immunity in models of inflammation (4–6), auto- Given the regulatory effect of SNS signaling in myeloid develop-
immunity (7, 8), and infection (9, 10). SNS activity has also been ment and the potently immunosuppressive effects of MDSCs, which
proven to play an important role in cancer, either by directly affecting are generated as a by-product of aberrant myeloid development, we
tumor cell survival (11, 12) or by regulating the functions of CD8+ investigated whether SNS activity influences tumor growth by reg-
T cells and natural killer (NK) cells that mediate tumor rejection ulating accumulation of MDSCs. We describe a mechanism by which
(13, 14). sympathetic control of myeloid development influences tumor im-
There are two major classes of ARs: -ARs and -ARs. The 2-AR munity by regulating accumulation of MDSCs that expand regula-
is the most ubiquitously expressed receptor on immune cells, but tory T cells (Tregs) and inhibit the antitumor immune response. We
each AR subtype (1-, 2-. 3-, 1-, and 2-ARs) has been reported show that ablation of sympathetic nervous signaling and the absence
to be expressed by at least one type of immune cell. The distinct pat- of a tonic -adrenergic maturation signal drive the accumulation of
tern of AR expression by immune cells shapes their capacity to re- immature, suppressive myeloid cells that are capable of suppressing
spond to SNS signals (3). tumor immunity and promoting tumor growth. Furthermore, we
During homeostasis, tonic sympathetic signaling has been shown show that these MDSCs secrete S100A8/A9, a highly abundant
to drive immune cell development and shape immune function. Ad- alarmin heterodimer and CD69 ligand expressed by cells of the neu-
renergic signaling regulates recruitment of leukocytes into tissue (15) trophil lineage (22, 23), that drives expansion of Tregs and thus com-
and can influence activity and differentiation of innate and adaptive pounds their immunosuppressive activity.
immune cells (4, 10, 13). For example, SNS activity has been proven
to play an important role in myeloid cell development and the abil-
ity of myeloid cells to mediate inflammation (5). RESULTS
Myeloid-derived suppressor cells (MDSCs) are generated from SNS tone regulates MDSC accumulation and
myeloid precursors as a result of aberrant differentiation and patho- tumor immunity
logical activation. The generation of MDSCs is a two-step process in To investigate the role of SNS tone on tumor immunity, CT26 colon
which a first group of signals (e.g., tumor-derived factors) drives the carcinoma cells were implanted into BALB/c mice that had been
expansion of immature myeloid precursors in the spleen or bone chemically sympathectomized by an intraperitoneal administration
marrow that subsequently get activated by a second group of signals of the neurotoxin 6-hydroxydopamine (6-OHDA). 6-OHDA is se-
[e.g., inflammatory cytokines, damage-associated molecular patterns lectively taken up by sympathetic terminals through the dopamine
(DAMP)] that induces them to acquire immunosuppressive functions and/or norepinephrine (NE) transporter when administered sys-
(16). They potently inhibit the antitumor immune response by a va- temically and subsequently destroys those nerve terminals and ab-
riety of mechanisms, including direct inhibition of the antitumor lates SNS signaling (24).
1
CT26 colon carcinoma tumors grew significantly faster in SNS-
Department of Immunology and Carole and Ray Neag Comprehensive Cancer Center, ablated BALB/c mice compared with vehicle-treated controls (Fig. 1,
University of Connecticut School of Medicine, Farmington, CT, USA. 2Department of
Computer Science and Engineering, University of Connecticut, Farmington, CT, USA. A and B). The effect of SNS ablation on tumor growth was dependent
*Corresponding author. Email: nevin@uchc.edu (J.T.N.); srivastava@uchc.edu (P.K.S.) on CD8+ T cells, because the effect of 6-OHDA was not observed
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 1 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
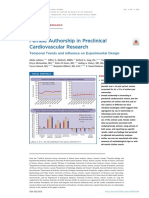
Fig. 1. Ablation of SNS results in accumulation A B 3000
Vehicle + αKLH 3000
6-OHDA + αKLH
of MDSCs and increased rate of tumor growth. 2000
Vehicle + αCD8a 2000 2000
(A) Summary tumor growth curves of wild-type
Tumor volume (mm 3)
Tumor volume (mm3)
6-OHDA + αCD8a ns, P = 0.3201
BALB/c mice treated with anti-CD8 or isotype 1500 Vehicle + αKLH
1000 1000
control antibody followed by treatment with 6-OHDA + αKLH 0 0
either 6-OHDA or vehicle control and tumor chal- 1000 0 10 20 30 40 0 10 20 30 40
Vehicle + αCD8a 6-OHDA + αCD8a
lenge with 3.5 × 105 CT26 colon carcinoma cells.
3000 3000
****P < 0.0001
Statistical analysis of tumor growth curves up to 500 2000 2000
day 24 was performed by two-way ANOVA, n = 8
1000 1000
0
to 10 mice per group. (B) Individual tumor growth
0 10 20 30
curves for each treatment group in (A). (C to Days after tumor challenge
0
0 10 20 30 40
0
0 10 20 30 40
F) Quantification of flow cytometric analysis of Days after tumor challenge
tumor-infiltrating (C and E) or splenic (D and F)
MDSCs from 6-OHDA–treated or vehicle-treated
C Tumor D Spleen E Tumor F Spleen G
% CD11b+ Ly6ChiLy6G- (of CD45 + )
% CD11b+ Ly6ChiLy6G- (of CD45 + )
% CD11b+ Ly6Cint Ly6G+ (of CD45 + )
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
% CD11b+ Ly6Cint Ly6G+ (of CD45 + )
60 2.0 8
CT26 tumor–bearing mice dissected 6 days af-
30 **P = 0.0016
Avg. tumor diameter (mm)
*P = 0.0117 8 ns, P = 0.6770
55 **P = 0.0049
***P = 0.0007
ter tumor challenge. Gating strategy in fig. S3. 6
50
1.5 6
20
(G) Quantification of average tumor diameter 45 1.0 4
4
6 days after CT26 tumor challenge from mice in 10 40
(C) to (F). Statistical analysis by two-tailed t test, 2
35
0.5 2
n = 7 mice per group. (H to K) Division index of 0 0 30 0.0 0
CFSE-labeled CD8+ T cells from a naïve wild-type
Vehicle 6-OHDA Vehicle 6-OHDA Vehicle 6-OHDA Vehicle 6-OHDA Vehicle 6-OHDA
BALB/c mouse after 72 hours of CD3/CD28
H I
stimulation and coculture with sorted CD3−CD19− CD11b+ CD11b+Ly6G+
Division index (of CD8 +CFSE+)
Division index (of CD8 +CFSE+)
CD11b+ cells (H), CD3−CD19−CD11b+Ly6CintLy6G+ 3 **
****
3
****
cells (I), CD3−CD19−CD11b+Ly6ChiLy6G− cells (J),
ns
****
*** ****
ns
or CD3−CD19−CD11b+Ly6C−Ly6G− cells (K) from
****
** ***
2 ** 2 ****
the spleen of 6-OHDA–treated or vehicle control– ns
** ns
treated CT26-bearing wild-type BALB/c mouse
*
21 days after tumor challenge. Statistical analysis 1 1
by two-way ANOVA with Sidak’s test for multiple ns
comparisons, n = 3 replicate wells per group, ex-
cept for 1:0.125 condition in SNS-ablated mice 0 0
1:0 1:0.5 1:0.25 1:0.125 1:0 1:0.5 1:0.25 1:0.125
in (K), which has only one data point. Data are CD8 :CD11b ratio + +
CD8+:CD11b+Ly6G+ ratio
representative of at least two independent ex- Vehicle 6-OHDA No CD11b+ Vehicle 6-OHDA No CD11b+
periments. ns, not significant. ns
CD11b+Ly6Chi CD11b+Ly6G− Ly6C−
Division index (of CD8 +CFSE+)
Division index (of CD8 +CFSE+)
J 3 K 3 ns
*
ns
****
*** *
**** ns
**** ns
**** *** **
when tumors were implanted in mice 2 *** 2
that had been depleted of CD8+ T cells ns
using an anti–CD8-depleting antibody 1 ns ns 1
(Fig. 1, A and B). The effect of SNS ab-
lation on the growth of MethA fibro-
sarcoma cells in BALB/c mice and MC38 0
1:0 1:0.5 1:0.25 1:0.125
0
1:0 1:0.5 1:0.25 1:0.125
colon carcinoma cells in C57BL/6 mice + + hi
CD8 :CD11b Ly6C ratio CD8+:CD11b+Ly6G− Ly6C− ratio
was also tested; both tumors grew faster Vehicle 6-OHDA No CD11b+ Vehicle 6-OHDA No CD11b+
in SNS-ablated mice compared with con-
trols (fig. S1). Neither CT26 nor MethA
tumor cells expressed any transcripts for genes encoding the NE detected (Fig. 1G), indicating that changes in MDSC accumulation
(Slc6a2) or dopamine (Slc6a3) transporter, indicating that they are precede any difference in tumor growth.
unable to respond to 6-OHDA directly (fig. S2A). Furthermore, none Phenotypic identification of MDSCs remains difficult because of
of the tumor lines expressed biologically significant levels of RNA a lack of uniquely identifiable surface markers (25). Thus, the sup-
for any ARs (fig. S2A), and culturing CT26 cells in the presence of pressive functions of myeloid cells in SNS-ablated tumor-bearing
NE or specific adrenergic agonists did not affect cell expansion (fig. mice were evaluated. CD11b+ cells isolated from the spleen of SNS-
S2B) or viability (fig. S2C), indicating that these tumor cells do not ablated tumor-bearing mice were more suppressive than those from
respond directly to SNS signaling. control-treated mice, as evidenced by their ability to suppress CD8+
Tumor-bearing SNS-ablated mice exhibited significantly increased (Fig. 1H) and CD4+ (fig. S4A) T cell proliferation in response to
proportions of both CD11b+Ly6CintLy6G+ polymorphonuclear cells CD3/CD28 stimulation. To test whether this difference is due to
and CD11b+Ly6ChiLy6G− monocytic cells in the tumor (Fig. 1, a change in the suppressive activity of MDSCs on a per-cell basis or
C and E) and in the spleen (Fig. 1, D and F) 6 days after tumor chal- an overall shift of the myeloid compartment toward an immuno-
lenge. These cells are phenotypically consistent with polymorpho- suppressive phenotype, subpopulations of CD11b+ myeloid cells were
nuclear (PMN)- and M-MDSCs (monocytic MDSCs), respectively. sorted from tumor-bearing SNS-ablated or control mice, and their
At this time point, a significant difference in tumor size was not suppressive activity was measured. The sorted CD11b+Ly6CintLy6G+
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 2 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
PMN-MDSC population from SNS-ablated mice exhibited signifi- 1 cells were actively dividing, as evidenced by their differential up-
cantly enhanced suppressive activity only at the 1:0.25 T cell:MDSC regulation of cell cycle–associated genes Mki67, Cks2, Cdk1, and
ratio compared with the corresponding populations from vehicle- Stmn1 (Fig. 3A). Thus, the transcriptional profile of these cells is
treated controls, whereas the sorted CD11b+Ly6ChiLy6G− M-MDSC consistent with that of the unipotent pre-neutrophil (preNeu) pro-
population from SNS-ablated mice was not significantly more sup- genitor cell immediately downstream of the granulocyte-monocyte
pressive than controls (Fig. 1, I and J), indicating that the heightened progenitor (28, 29).
suppressive activity of the myeloid compartment in SNS-ablated Flow cytometric analysis of the spleen of tumor-bearing SNS-
mice shown in Fig. 1H is likely due to the increased proportion of ablated mice confirmed the finding that immature PMNs accumulate
MDSCs (as shown in fig. S4E) rather than augmented suppressive in the absence of SNS signaling. A smaller proportion of CD11b+
activity of MDSCs on a per-cell basis. CD11b+Ly6C−Ly6G− cells did Ly6CintLy6G+ cells expressed CD101 (fig. S9A) and CXCR2 (fig. S9B),
not objectively suppress CD8+ T cell proliferation (Fig. 1K), but and a larger proportion of CD11b+Ly6CintLy6G+ cells highly ex-
CD8+ T cells cultured with CD11b+Ly6C−Ly6G− from SNS-ablated pressed Ki67 (fig. S9C) in SNS-ablated mice, indicating a relatively
mice were significantly less proliferative than T cells from vehicle- immature phenotype.
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
treated mice, potentially indicating the presence of another suppres- Adrenergic signaling has been shown to drive maturation of my-
sive population of Ly6C−Ly6G− cells in these cocultures. eloid cells in diabetic mice and humans (5). To test whether the
removal of an adrenergic maturation signal by SNS ablation was
Immature myeloid cells expand upon SNS ablation driving the accumulation of immature myeloid cells in cancer, we
The characteristics of the myeloid compartment of SNS-ablated tumor- evaluated the maturity of PMNs that accumulated in the spleen of
bearing mice were further investigated by single-cell RNA sequenc- SNS-ablated tumor-bearing mice by analysis of their RNA velocity,
ing. Single non–T and non–B cells (CD3−CD19−) were sorted from which enables the inference of the developmental trajectories of single
the spleen of SNS-ablated or vehicle control–treated CT26 tumor– cells (30). Genes known to be expressed early in neutrophil develop-
bearing mice and captured using the 10× Chromium droplet-based ment (e.g., Cybb and Nox2) were found to have positive velocity in
microfluidic system. After removal of NK subsets (fig. S5), the my- immature neutrophils (cluster 3), preNeu (cluster 1), and PMN-
eloid compartment clustered into eight distinct classifications (Fig. 2, MDSC (cluster 4; Fig. 3B), indicating up-regulation of these genes
A to C; selected differentially expressed (DE) gene expression in fig. in these populations. Likewise, genes known to be preferentially
S7). Two distinct clusters of polymorphonuclear neutrophil–like cells, expressed by more mature neutrophils (e.g., Csf3r and Cd101) were
clusters 4 and 5, were identified (by >99% of cells in these clusters found to have positive velocity in mature neutrophils (cluster 2) but
expressing both S100a8 and S100a9; fig. S6). These two clusters were not by more immature populations (Fig. 3B).
downloaded, reanalyzed, and characterized into five distinct popu- Cell-specific velocities were calculated and projected on the
lations (Fig. 2D) for further analysis, as described below. t-stochastic neighbor embedding (t-SNE) of the PMNs from Fig. 2
The PMN phenotype was conspicuously altered in SNS-ablated (D to F) using local transition probabilities based on the correlation
mice. PMN cluster 4 was observed to expand from 10.71 to 21.01% between the velocity and gene expression states of the cells as described
of PMNs upon SNS ablation in tumor-bearing mice (Fig. 2E). This in (30) (Fig. 3, C to E). This analysis of cell-specific RNA velocity allows
cluster was defined by relatively high expression of S100a8 and S100a9, for the quantitative modeling of cell fates (30). This analysis predicts
high expression of Cybb (Nox2), differential up-regulation of tran- that PMN-MDSCs and mature neutrophils developed via divergent
scription factors Stat3 and Cebpb, and differential up-regulation of pathways from the preNeu cluster (PMN cluster 1). This is consistent
the suppressive cytokine Tgfb1 (Fig. 2F)—all of which are associated with a recent report that shows mature neutrophils and PMN-MDSC
with the PMN-MDSC phenotype (18, 26, 27). arise from a common precursor in the spleen in tumor-bearing mice
PMN clusters 3 and 5 were also observed to expand in SNS- (31). Together, our analysis of single-cell RNA expression and RNA
ablated mice (Fig. 2E). These clusters were defined by high expres- velocity suggests that the SNS plays a critical role in controlling myeloid
sion of S100a8, S100a9, and Nox2, relatively low expression of Stat3 maturation and accumulation of MDSCs in a tumor-bearing host.
and Tgfb1, and relatively high expression of the transcription factor Immature neutrophils, mature neutrophils, and preNeu were also
Cebpe (Fig. 2F and fig. S8). Their transcriptional signature is consistent identified in the bone marrow of tumor-bearing mice by single-cell
with immature, developing neutrophils (28) but is distinct from the RNA sequencing (fig. S10), as has been previously described (28), and
PMN-MDSC signature expressed by cluster 4. their developmental trajectory was assessed by RNA velocity (fig. S11).
PMN cluster 2 was observed to be present at a much lower pro- However, the proportions of each PMN subpopulation were essen-
portion in SNS-ablated mice compared with controls (Fig. 2E). Cells tially unchanged in the bone marrow of SNS-ablated mice as com-
in this cluster were defined by expression of neutrophil markers pared with controls (fig. S10, D to F). This suggests that sympathetic
Cxcr2 and Csf3r [granulocyte colony-stimulating factor (G-CSF) regulation of myeloid development in the spleen, but not in the bone
receptor], inflammatory cytokines Il1b [interleukin-1 (IL-1)] and marrow, affects the accumulation of PMN-MDSCs in tumor-bearing
Il1f9 (IL-36), and the mature neutrophil marker Cd101; thus, their mice. This is also consistent with a recent report that indicates that
transcriptional signature is consistent with that of mature neutro- the spleen is the primary site of MDSC generation in a tumor-bearing
phils (fig. S8) (28). host (31) and another that shows that sympathetic activity regulates
PMN cluster 1 was observed to markedly expand in SNS-ablated myelopoiesis in the spleen but not in the bone marrow (5).
mice, from 6.86 to 20.82% of PMNs (Fig. 2E). This cluster not only
shared some of the characteristics of immature PMN clusters 3 and 5 - and -AR signaling elicit opposing effects on tumor
but also was observed to express genes encoding primary and sec- immunity and development of MDSCs
ondary granule proteins (e.g., Mpo, Elane, and Camp) and tran- NE produced by sympathetic neurons that innervate lymphoid or-
scription factors Cebpe and Gfi1 (fig. S8). In addition, PMN cluster gans elicits its cellular effects by binding ARs expressed by immune
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 3 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
Cluster that fewer PMNs expressed CD101 (Fig. 4A)
A C 8
7 and more PMNs expressed intermediate
6 and high levels of Ki67 (Fig. 4B) upon cul-
5 ture with the -AR agonist, indicating a
Percent of cells analyzed
4
relatively immature phenotype compared
1 7 6 3 2 8 5 4 with controls. Because immaturity is one
B Cluster Classification Selected DE genes 3
of the hallmarks of the PMN-MDSC
1 Monocyte/M-MDSC Ccr2, S100a4, Csf1r, Fcgr3, Cebpb
2 Conventional DC Cd74, H2-Aa, Itgax, H2-Oa, Cd86, Cd80
2
phenotype, this finding supports pub-
3 Plasmacytoid DC Siglech, Cox6a2, Klk1 lished data demonstrating that -AR sig-
4
5
Neutrophil-like cell 1 S100a8, S100a9, Cd177, Ltf, Ly6g, Camp
Neutrophil-like cell 2 Csf3r, Cxcr2, Il1f9, Nlrp12, Cd101
naling promotes MDSC accumulation (34).
6 Erythroblast-like cell Tfrc, Ermap, Car1, Car2, Hba-a1 1 2-AR signaling had an opposite effect
7 Basophil Mcpt8, Fcer1a, Il4, Il6, Csf1 on the generation of MDSCs. We observed
8 Macrophage Mertk, Axl, C1qa, C1qb, C1qc, Cd163
Vehicle 6-OHDA that more CD11b+S100A9+Ly6G+ PMNs
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
Subclustering of neutrophil-like clusters 4 and 5 PMN expressed CD101 and low levels of Ki67
Cluster 3
E cluster
and fewer PMNs expressed high levels
D Immature
neutrophils
5
of Ki67, indicating a relatively mature
Cluster 5
4
Immature
Cluster 1 phenotype compared with controls (Fig. 4,
neutrophils
Pre - A and B). 1-AR signaling caused a mod-
Percent of cells analyzed
neutrophils
3 est reduction in CD101 expression on
PMNs but showed no effect on their pro-
liferation (Fig. 4, A and B). Thus, ago-
2 nism of 1- and 2-ARs has divergent
effects on the development of MDSCs.
In addition to its expression by neu-
Cluster 4 1
trophils and PMN-MDSC, S100A9 is ex-
Cluster 2
Mature
PMN -
MDSC
pressed by hematopoietic precursors of
neutrophils Vehicle 6-OHDA neutrophils and monocytes (28, 35). We
F S100a9 Cebpb Stat3 Tgfb1 evaluated the proliferation of CD11b+
S100A9+Ly6G− cells after culture with
specific - or -AR agonists (Fig. 4C).
(scaled log-transformed
We found a higher proportion of Ki67int
Gene expression
avg. expression)
and lower proportion of Ki67lo cells upon
culture with the -AR agonist, indicat-
ing that -AR stimulated the prolifera-
tion of myelopoietic cells, consistent with
a previous report (5). However, we also
1 2 3 4 5 1 2 3 4 5 1 2 3 4 5 1 2 3 4 5
Cluster ID found a lower proportion of Ki67int and
Fig. 2. SNS exerts tonic control over suppressive activity of PMNs in a tumor-bearing host. (A to C) Spleens were
higher proportion of Ki67lo cells upon
harvested 18 days after tumor challenge of SNS-ablated or vehicle-treated mice, and CD3−CD19− cells were fluorescence culture with the 2-AR agonist, indicat-
activated cell sorted and captured using the 10× Chromium droplet-based microfluidic system. (A and B) Classifica- ing that 2-AR agonism inhibited the
tion of non-NK cells by TF-IDF–based clustering (45) into eight clusters. Differential expression analysis performed by proliferation of myelopoietic cells. Cul-
t test with Welch (or Satterthwaite) approximation and 0.95 CI, genes with log2 fold change of ≥2 and P ≤ 0.01 were ture with the 1-AR agonist resulted in
considered to be differentially expressed. (C) Clustering breakdown by sample as a percentage of total cells analyzed. a modest reduction of Ki67lo cells but no
(D) Subanalysis of PMN clusters 4 and 5 (1752 cells) into five distinct clusters, plotted as three-dimensional t-SNE. difference in the Ki67int or Ki67hi popu-
(E) Subclustering breakdown of PMNs by sample as a percentage of total cells analyzed. (F) Violin plots of gene ex- lations. Thus, signaling through 2- or
pression for selected genes in PMN clusters. Single-cell RNA sequencing experiment was performed one time. DC, -ARs elicits a reciprocal effect on the
dendritic cell.
maturation of PMNs and the prolifera-
tion of myelopoietic precursor cells.
(or nonimmune) cells that are in close proximity to the sympathetic Previous studies have shown that systemic blockade of -ARs
nerve terminal (32). NE binds with high affinity to -ARs, with mod- leads to improved immune-mediated tumor control (13); our exper-
erate affinity for the 1-AR and with poor affinity for the 2-AR (33). iments (Fig. 4D) confirm this phenomenon. CT26 colon carcinoma
The cellular mechanism of adrenergic regulation of MDSC devel- cells were implanted into BALB/c mice treated daily with propran-
opment was investigated in vitro. MDSCs were generated by culturing olol (10 mg/kg; a nonselective -AR antagonist) starting 3 days be-
bone marrow cells from BALB/c mice in CT26 tumor–conditioned fore tumor challenge. This treatment had a significant inhibitory
media (CT26-TCM) supplemented with granulocyte-macrophage effect on tumor growth (Fig. 4D), as reported previously (13, 34).
(GM)–CSF in the presence of isoproterenol (a nonselective -AR However, the role of -AR signaling on tumor immunity is un-
agonist), phenylephrine (an 1-AR agonist), or clonidine (an 2-AR clear. To characterize the effect of -AR signaling on tumor growth,
agonist). We analyzed the maturity of CD11b+S100A9+Ly6G+ PMN CT26 tumors were implanted into mice treated daily with a combi-
cells after 6 days by their expression of CD101 and Ki67. We observed nation of phentolamine (10 mg/kg; a nonselective -AR antagonist)
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 4 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
A Mki67 Cks2 Cdk1 Stmn1
alone (Fig. 4, D and E). This suggests that
-AR blockade contributes to enhance-
ment of tumor growth, similar to the
(scaled log-transformed
phenotype observed upon sympathetic
Gene expression
avg. expression)
denervation with 6-OHDA. These results
demonstrate that -adrenergic signaling
plays an important role in tumor growth
and that the immunosuppressive effects
1 2 3 4 5 1 2 3 4 5 1 2 3 4 5 1 2 3 4 5 of SNS ablation result from a lack of
Cluster ID
-adrenergic signaling.
B Cybb Ltf
SNS tone regulates
immune homeostasis
Unspliced (u)
Unspliced (u)
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
To test the effect of SNS tone on myeloid
development at homeostasis (i.e., in non–
Spliced (s) Spliced (s) tumor-bearing mice), we treated naïve
Csf3r Cd101
BALB/c mice with 6-OHDA to ablate
the SNS and measured the phenotype
Unspliced (u)
Unspliced (u)
and maturity of myeloid cells. SNS-
ablated mice exhibited significantly in-
creased proportions of both CD11b+
Ly6CintLy6G+ polymorphonuclear cells
Spliced (s) Spliced (s)
RNA velocity Gene expression RNA velocity Gene expression
and CD11b +Ly6C hiLy6G − monocytic
C D Start point cells in the spleens (Fig. 5A) of tumor-
Cluster 5 distribution free mice, similar to what was observed
Immature
neutrophils Cluster 1
in tumor-bearing mice. A smaller pro-
Pre-
neutrophils
portion of CD11b+Ly6CintLy6G+ PMNs
from SNS-ablated mice were observed
Cluster 2 Cluster 3 to express CD101, a marker of mature
Mature
neutrophils
Immature
neutrophils
neutrophil phenotype, than controls. In
addition, a higher proportion of CD11b+
Ly6CintLy6G+ PMNs from SNS-ablated
E End point mice were observed to be actively pro-
distribution liferating as assessed by high expression
of Ki67, a characteristic of immature neu-
trophil precursors, than controls (Fig. 5B).
Cluster 4
PMN- Together, these data suggest that the
MDSC
absence of sympathetic nervous signal-
ing at homeostasis disrupts neutrophil
maturation and promotes the accumu-
lation of immature myeloid cells.
Fig. 3. Ablation of SNS interrupts neutrophil development and promotes accumulation of immature myeloid Myeloid cells from SNS-ablated mice
cells in a tumor-bearing host. (A) Violin plots of gene expression for selected cell cycle–associated genes in PMN were suppressive even in the absence of
clusters. (B) Left: Abundance of spliced (s) and unspliced (u) mRNAs for selected genes colored as in Fig. 2 (D to F).
a tumor. Sorted CD11b+ cells from the
Middle: RNA velocity field. Right: Gene expression profiles. Red denotes positive velocity, which correlates with gene
spleen of SNS-ablated mice were more
up-regulation. Blue denotes negative velocity, which correlates with gene down-regulation. (C) Two-dimensional
t-SNE embedding showing PMNs from Fig. 2 (D to F). Individual cells are colored by clusters designated in Fig. 2E.
suppressive than those from control-
Arrows show extrapolated cell state projection on t-SNE embedding summarized as velocity field calculated on a treated mice, as evidenced by their abil-
grid. (D and E) Density distribution of differentiation start points (D) and end points (E) as determined by RNA velocity. ity to suppress CD4+ T cell proliferation
Single-cell RNA sequencing experiment was performed one time. in response to CD3/CD28 stimula-
tion (Fig. 5C). Sorted CD11b+Ly6Cint
Ly6G+ cells suppressed T cell prolifer-
and propranolol (10 mg/kg) starting 3 days before tumor challenge. ation, but their suppressive activity was not affected by SNS abla-
Treatment with phentolamine alone was not attempted because an- tion (Fig. 5D). Sorted CD11b+Ly6ChiLy6G− monocytic cells also
tagonism of the 2-AR also inhibits a negative feedback mechanism suppressed T cell proliferation and were more suppressive in
of NE release by the presynaptic neuron, thereby increasing the local SNS-ablated mice only at the 1:0.125 T cell:monocytic cell ratio
amount of NE available and potentially leading to increased engage- (Fig. 5E). CD11b+Ly6C−Ly6G− cells did not suppress T cell prolifer-
ment of -ARs. Dual blockade of - and -ARs resulted in significantly ation. Thus, the myeloid compartment in tumor-free mice becomes
increased rate of CT26 tumor growth (Fig. 4F) and decreased sur- more suppressive in the absence of functional sympathetic nervous
vival of tumor-bearing mice (Fig. 4G), as compared with propranolol signaling.
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 5 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
A **** B C ***
leading to their increased proportion in
***
SNS-ablated mice.
(% of CD11b + S100A9+ Ly6G+ )
50 100 **** 80
% of CD11b+ S100A9+ Ly6G−
****
% of CD11b+ S100A9+ Ly6G+
** ns Control
ns **
40 80
**
***
Isoproterenol Tregs have been described to potently
60 ns Clonidine
suppress the antitumor immune response,
CD101+ Ki67lo
Phenylephrine
30 60
40
and cross-talk between MDSCs and Tregs
20 40 *** can synergistically inhibit tumor im-
** * ns
10 20
ns
**
ns
20 ns
ns
munity (36, 37). For example, MDSCs
have been demonstrated to induce the
0 0
Ki67lo Ki67int Ki67hi
0
Ki67lo Ki67int Ki67hi
development and expansion of Tregs in
a tumor-bearing host (17), amplifying
e
ep e
l
Ph l o n o l
ro tro
in
in
n
hr
en id
re
op n
Is Co
te
the suppression of antitumor immunity.
yl
C
The role of MDSCs in SNS ablation–
D E elicited expansion of Tregs was examined.
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
800 100
Naïve mice were depleted of Gr-1+ cells
Tumor volume (mm3)
Propranolol
using an anti–Gr-1 antibody (fig. S14),
Percent survival
600
PBS
and SNS ablated via peripheral admin-
400 50 istration of 6-OHDA. Whereas SNS ab-
*P = 0.0195 lation induced Treg proliferation in mice
ns, P = 0.5833
Propranolol treated with an isotype control antibody,
200 PBS SNS ablation had no effect on Treg pro-
liferation in mice that lacked Gr-1+ cells
0 0
0 5 10 15 20 25 0 20 40 (Fig. 6D), indicating that Treg expan-
Days after tumor challenge Days after tumor challenge sion upon SNS ablation is dependent on
Gr-1+ cells.
F 800 G 100
Propranolol +
MDSC-derived S100A8/A9 induces
Tumor volume (mm3)
phentolamine
Treg expansion in SNS-ablated mice
Percent survival
600
PBS
Neutrophils and MDSCs express and
**P = 0.0018
400 50 can secrete extremely high levels of the
calcium-binding proteins S100A8 and
****P < 0.0001 PBS
200
S100A9 (18, 22). These proteins are high-
Propranolol +
phentolamine
ly abundant in cells of the neutrophil
lineage; their expression is induced im-
0 0
0 5 10 15 20 25 0 20 40 mediately downstream of the granulocyte-
Days after tumor challenge Days after tumor challenge monocyte progenitor during neutrophil
development (28), and together, they
Fig. 4. - and -AR signaling elicit opposing effects on tumor immunity and development of MDSCs. (A) Quan-
make up about 45% of all cytoplasmic
tification of maturity by CD101 expression of CD11b+S100A9+Ly6G+ PMN generated in vitro upon culture with spe-
cific AR agonists. Statistical analysis by one-way ANOVA with Dunnett’s test for multiple comparisons with a single
proteins in mature neutrophils (22).
pooled variance. (B and C) Quantification of proliferation by Ki67 expression of either CD11b+S100A9+Ly6G+ (B) or S100A8 and S100A9 come together to
CD11b+S100A9+Ly6G− cells (C) generated in vitro upon culture with a specific AR agonist. Statistical analysis by two- form a heterodimer that elicits diverse
way ANOVA with Dunnett’s test for multiple comparisons, n = 3 replicate wells per condition. Gating strategy in fig. cellular effects to maintain neutrophils
S12. Isoproterenol (nonspecific -AR agonist); clonidine (2-AR agonist); phenylephrine (1-AR agonist). (D) Summary at homeostasis and initiate their migra-
tumor growth curve (up to day 21) of wild-type BALB/c mice treated daily with propranolol (10 mg/kg) or vehicle tion and phagocytic activity during an
control (PBS) and intradermally injected with 3.5 × 105 CT26 colon carcinoma cells. (E) Survival analysis of (D). active infection. S100A8/A9 can also
(F) Summary tumor growth curve (up to day 21) of wild-type BALB/c mice treated daily with a combination of pro- be secreted; the effects of extracellular
pranolol (10 mg/kg) and phentolamine (10 mg/kg) or vehicle control (PBS) and intradermally injected with 3.0 × 105
S100A8/A9 are thought to be predomi-
CT26 colon carcinoma cells. (G) Survival analysis of (D). Statistical analysis of tumor growth curves up to day 21 was
nantly proinflammatory and are elicited
performed by two-way ANOVA, n = 8 to 10 mice per group. Survival analysis by log-rank (Mantel-Cox) test, n = 8 to
10 mice per group. Data are representative of at least two independent experiments, except for (D) and (E), which
upon binding of the heterodimer to ei-
was performed once. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. ther RAGE (receptor for advanced glyca-
tion endproducts) or Toll-like receptor 4
(TLR4) on target cells (22).
In addition to its effect on myeloid cells, SNS ablation also influ- We observed that depletion of Gr-1+ cells also led to the deple-
enced the composition of the T cell compartment at homeostasis. tion of virtually all cells that express S100A9 in the spleen (Fig. 6E).
Specifically, SNS ablation induced the expansion of Tregs in non– Furthermore, we observed that the serum level of S100A8/A9 het-
tumor-bearing mice. 6-OHDA treatment induced an increased pro- erodimer was greatly increased in SNS-ablated mice (Fig. 6F), con-
portion of Tregs in the spleen of sympathectomized wild-type mice cordant with the observed increase in PMNs (Fig. 5A).
(Fig. 6, A and B), consistent with a previous report (8). Elevation of In addition to its classical receptors RAGE and TLR4, the
Treg proportion in SNS-ablated mice was long-lasting (fig. S13). S100A8/A9 heterodimer has been demonstrated to signal through
Tregs were observed to proliferate upon SNS ablation (Fig. 6C), likely CD69 expressed on the surface of T cells (23), which results in
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 6 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
A Ly6G + Ly6C hi B CD101 Ki67
Furthermore, the presence of S100A8/
% CD11b+ Ly6Cint Ly6G+ (of live cells)
% CD101+ (of CD11b + Ly6Cint Ly6G+ )
% CD11b+ Ly6ChiLy6G− (of live cells)
% Ki67hi (of CD11b + Ly6Cint Ly6G+ )
5 1.5 100 20 A9 was able to compensate for a lack of
***P = 0.0008 ****P < 0.0001 *P = 0.0247 *P = 0.0287 IL-2 to promote iTreg differentiation and
4 95
1.0
15 proliferation under IL-2–scarce condi-
3 90
10
tions. Even in the presence of an IL-2–
2 85 blocking antibody, the proportion (Fig. 6,
0.5
1 80
5 J and L) and the absolute number (fig.
S14) of iTregs were significantly increased
0 0.0 75 0
Vehicle 6-OHDA Vehicle 6-OHDA Vehicle 6-OHDA Vehicle 6-OHDA
in the presence of S100A8/A9. Further-
more, iTregs are significantly more pro-
liferative upon culture with S100A8/A9
C D
Division index (of CD4+ CFSE+ )
Division index (of CD4+ CFSE+ )
3
ns
3
****
(Fig. 6, K and M), indicating that Tregs
**** are able to directly respond to S100A8/
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
****
* ****
**** ****
ns
*
***
***
A9. These data demonstrate a mecha-
2 **** *** ns
2 ns nism by which MDSC-derived S100A8/
ns A9 can drive expansion of Tregs by mimick-
ing IL-2 signaling and illustrate a means
1 1 ns of MDSC/Treg interplay that can suppress
antitumor immunity.
0 0
1:0 1:0.5 1:0.25 1:0.125 1:0 1:0.5 1:0.25 1:0.125
+
CD4 :CD11b ratio +
CD4+ :CD11b+ Ly6C int Ly6G+ ratio DISCUSSION
Vehicle 6-OHDA No CD11b+ These results describe a regulatory frame- Vehicle 6-OHDA No CD11b+
work in which sympathetic tone controls
Division index (of CD4+ CFSE+ )
Division index (of CD4+ CFSE+ )
E 3 F 3
****
** the development of innate and adaptive
***
ns
**** ** immune cell populations at homeostasis
**** *
****
**** ** ns ns and influences their activity in disease.
2 ***
***
2 They also demonstrate a mechanism by
which sympathetic nervous control of
ns ns
*** immune development influences anti-
1 1
tumor immunity.
Previous studies have established that
adrenergic signaling to granulocyte-
0 0
1:0 1:0.5 1:0.25 1:0.125 1:0 1:0.5 1:0.25 1:0.125 monocyte progenitor cells can promote
+ +
CD4 :CD11b Ly6C Ly6G ratio hi −
CD4+ :CD11b+ Ly6G− Ly6C − ratio their proliferation and affect their dif-
Vehicle 6-OHDA No CD11b+ Vehicle 6-OHDA No CD11b+ ferentiation into mature myeloid cells
Fig. 5. Ablation of SNS promotes accumulation of immature, suppressive myeloid cells in a tumor-naïve host. capable of mediating inflammation (5).
(A) Quantification of flow cytometric analysis of splenic CD11b+Ly6CintLy6G+ cells and CD11b+Ly6ChiLy6G− cells from Our results establish that, in the absence of
6-OHDA–treated or vehicle-treated BALB/c mice 10 days after initiation of chemical denervation. (B) Analysis of neu- a tonic adrenergic maturation signal, im-
trophil maturity as measured by CD101 and Ki67 expression on splenic CD11b+Ly6CintLy6G+ cells from 6-OHDA– mature myeloid cells accumulate and are
treated or vehicle-treated mice. Statistical analysis by two-tailed t test, n = 5 mice per group. (C to F) Division index of primed to acquire a suppressive MDSC
CFSE-labeled CD4+ T cells from naïve wild-type BALB/c mice after 72 hours of CD3/CD28 stimulation and coculture phenotype. We further show that the dis-
with sorted CD3−CD19−CD11b+ cells (C), CD3−CD19−CD11b+Ly6CintLy6G+ cells (D), CD3−CD19−CD11b+Ly6ChiLy6G−
− − + − −
ruption of myeloid development in the
cells (E), or CD3 CD19 CD11b Ly6C Ly6G cells (F) from the spleen of 6-OHDA–treated or vehicle control–treated
absence of tonic SNS signaling leads to
wild-type BALB/c mouse 10 days after initiation of chemical denervation. Statistical analysis by two-way ANOVA with
Sidak’s test for multiple comparisons, n = 3 replicate wells per group. Data are representative of at least two indepen-
accumulation of MDSCs and impaired
dent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. tumor immunity.
Recent studies have demonstrated that
2-AR–mediated signaling promotes the
phosphorylation of signal transducers and activators of transcrip- accumulation of MDSCs and the acquisition of a suppressive pheno-
tion 5 (STAT5) (38). We hypothesized that secretion of S100A8/A9 type by myeloid cells (34). Although our conclusion that the absence
by MDSCs might induce expansion of Tregs by inducing phospho of tonic SNS input promotes MDSC accumulation may appear con-
rylation of STAT5 and mimicking IL-2 signaling in T cells. tradictory, these findings are complementary. NE has a much higher
To test this, we induced Treg differentiation in vitro in the pres- affinity for -ARs than for -ARs and an extremely low affinity for
ence or absence of recombinant S100A8/A9 heterodimer. The pres- the 2-AR (33). Thus, we posit (fig. S16) that low-level tonic SNS sig-
ence of S100A8/A9 increased induced Treg (iTreg) differentiation as naling is likely to signal predominantly through -ARs, whereas en-
measured by the proportion of CD4+ T cells expressing FoxP3 after gagement of -ARs by NE is likely to require a stronger stimulus (or
3 days of culture (Fig. 6G). The presence of S100A8/A9 also in- to be mediated by systemic epinephrine, which has a higher affinity
creased the proliferation of iTregs as measured by the division index for -ARs than -ARs). Therefore, we propose that tonic produc-
of CD4+FoxP3+ cells (Fig. 6, H and I). tion of NE by sympathetic nerves that innervate lymphoid tissue
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 7 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
delivers an -adrenergic signal that is
A FoxP3+ B FoxP3+ C Ki67 D Treg proliferation required to drive myeloid maturation,
% Ki67+ (of CD3 + CD4+ FoxP3+ )
% Ki67+ (of CD3 + CD4+ FoxP3+ )
****P < 0.0001 ****P < 0.0001 40 *P = 0.0124
6 ***P = 0.0002 25 60
but excessive local production of NE
% CD4+ FoxP3+ (of live cells)
% FoxP3+ (of CD3 + CD4+ )
ns, P = 0.4547
20
30 or systemic production of epinephrine
4 40
15
20
during a stress response engages low-
2
10
20
affinity -ARs (which are more abundant
5
10 in immune cells) and causes myeloid
0 0 0 0
cells to become susceptible to acquir-
Vehicle 6-OHDA Vehicle 6-OHDA Vehicle 6-OHDA LTF-2 LTF-2 α Gr-1 α Gr-1
Vehicle 6-OHDA Vehicle 6-OHDA
ing suppressive functions. This idea is
E + F S100A8/A9 G iTreg
supported by our findings that 2-AR–
S100A9
25
100 mediated signaling antagonizes the ac-
***P = 0.0002 ****P < 0.0001
% S100A9+ (of live cells)
6000
% FoxP3+ (of total CD4+)
20
Serum S100A8/A9 (pg/ml) ***P = 0.0002 cumulation of immature MDSC and
80
promotes their maturation in vitro
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
15 4000
60 and that concurrent blockade of - and
10 40 -adrenergic signaling in vivo mirrors
ns, P = 0.8051 2000
5 20
the effect of SNS ablation and promotes
tumor growth.
0
LTF-2 LTF-2 α Gr-1 α Gr-1
0
Vehicle 6-OHDA
0
These results also reveal an indirect
l
mechanism by which sympathetic reg-
9
ro
l
ro
Vehicle 6-OHDA Vehicle 6-OHDA
A
nt
8/
nt
co
A
o
ulation of myeloid development affects
00
g c
H0
S1
re
T
iT
g +
H I J K adaptive immunity. Expansion of SNS-
re
iT
CFSE +CD4 +FoxP3 + **P = 0.0022
Proliferation index (of CD4+FoxP3+)
responsive MDSCs further induces the
Division index (of CD4+FoxP3+)
1.0 ***P = 0.0005
80 2.0
expansion of Tregs, thus amplifying their
**P = 0.0096
****P < 0.0001
% FoxP3+ (of total CD4+)
0.8
60
**P = 0.0025 1.8
***P = 0.0005 suppression of tumor immunity. Previ-
0.6 1.6
**P = 0.0036
ous studies have identified that MDSCs
40
0.4 1.4 facilitate Treg expansion and suppres-
0.2 20
1.2
sive function (37) and that depletion of
0.0
MDSCs results in a concurrent reduc-
0 1.0
iTreg control 0.0 0.1 1.0 0.0 0.1 1.0 tion in Treg numbers in tumor-bearing
9
l
ro
Concentration αIL-2 (µg/ml) Concentration αIL-2 (µg/ml)
8/
mice (39). This regulation occurs via
nt
iTreg + S100A8/A9
A
co
00
S1
g
Control +S100A8/A9 Control +S100A8/A9
several mechanisms; for example, MDSC-
re
iT
+
g
re
iT
L Control αIL-2, 0.1 µg/ml αIL-2, 1 µg/ml M Control αIL-2, 0.1 µg/ml αIL-2, 1 µg/ml
derived cytokines [e.g., transforming
growth factor– (TGF-) and IL-10]
iTreg promote Treg conversion and survival
+ (17), MDSC-derived chemokines at-
S100A8/A9
tract T regs to the tumor microenvi-
ronment (40), and cell-cell interactions
Gated on: CFSE +CD4 +FoxP3 + between MDSCs and Tregs via CD40/
CFSE
iTreg CD40L induce Treg expansion and pro-
control mote tolerance (41).
FoxP3
This study identifies the production
of S100A8/A9 by MDSCs as a regulator
CD4
of Treg activity and thus as a regulator of
Fig. 6. MDSC-derived S100A8/A9 induces Treg expansion in SNS-ablated mice. (A to C) Quantification of flow
adaptive tumor immunity. S100A8/A9
cytometric analysis of Treg proportion of live cells (A), Treg proportion of CD3+CD4+ cells (B), and Treg proliferation by
Ki67 expression (C) in the spleen of SNS-ablated or control mice 6 days after initiation of chemical denervation. Sta-
has been identified as a ligand of CD69
tistical analysis by two-tailed t test, n = 5 mice per group. (D) Quantification of Treg proliferation by Ki67 expression (23), which has been demonstrated to
from the spleen of SNS-ablated or control mice treated with either anti–Gr-1 antibody or isotype control. (E) Quanti- be a critical regulator of the immuno-
fication of S100A9+ cells in the spleen of SNS-ablated or control mice treated with either anti–Gr-1 antibody or iso- suppressive functions of Tregs (42, 43).
type control. Statistical analysis by one-way ANOVA with Tukey’s test for multiple comparisons with a single pooled The ligand of CD69 that mediates these
variance. (F) Quantification of S100A8/A9 heterodimer in the serum of 6-OHDA–treated or vehicle-treated mice by effects in Tregs is still unclear. We pro-
ELISA. Statistical analysis by two-tailed t test, n = 10 mice per group. (G) Quantification of iTreg induction after pose that the binding of S100A8/A9 to
72 hours of activation in the presence of TGF- with or without recombinant S100A8/A9 heterodimer. (H) Division CD69 and subsequent phosphoryla-
index of CD4+FoxP3+ iTreg differentiated with or without recombinant S100A8/A9 heterodimer. Statistical analysis by tion of STAT5 acts as a Treg survival/
two-tailed t test, n = 3 replicate wells per group. (I) Representative CFSE tracing of CFSE+CD4+FoxP3+ iTreg from (G)
differentiation/proliferation signal even
and (H). (J and L) Quantification of flow cytometric analysis (J) and representative examples (L) of iTreg differentiation
from naïve CD4+ T cells in the presence of titrated IL-2 antibody with or without recombinant S100A8/A9 heterodi-
in limiting quantities of IL-2. Therefore,
mer. (K and M) Quantification of CD4+FoxP3+ iTreg proliferation index (K) and representative CFSE tracing (M) in the we propose that MDSC-induced expan-
presence of titrated anti–IL-2 antibody with or without recombinant S100A8/A9 heterodimer. Statistical analysis by sion of Tregs via secretion of S100A8/A9
two-way ANOVA with Sidak’s test for multiple comparisons, n = 3 replicate wells per group. Data are representative is a further mechanism of synergistic
of at least two independent experiments. TH0, T helper cell 0. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. immunosuppression in cancer that can
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 8 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
be pharmacologically targeted to improve response to immuno- Sigma-Aldrich), or vehicle control (PBS) daily starting 3 days before
therapy and improve patient outcomes. tumor challenge. CT26 colon carcinoma (3 × 105 to 3.5 × 105) cells
were intradermally implanted into the right flank immediately after
day 0 AR antagonist injection. Daily injections were continued for
MATERIALS AND METHODS the duration of the experiment. Tumor growth was measured in
Study design two perpendicular dimensions by caliper at least twice weekly. Tu-
The objective of this study was to characterize the role of sympa- mor volume was calculated using the formula V = (l × w2)/2. Mice
thetic nervous activity on the immune response to cancer. All ex- were euthanized when average tumor diameter reached ≥15 mm.
periments were performed on BALB/cJ mice purchased from the For survival analysis, end point was considered to be when average
Jackson laboratory. Researchers were not blinded to experimental tumor diameter reached ≥15 mm or a maximum of 50 days after
groups. Each experiment was performed at least two times, with the tumor challenge.
exception of single-cell RNA sequencing, Fig. 4 (D and E) and figs.
S1, S2, S9, and S13. CD8+ cell depletion in Fig. 1A and fig. S1A was Analysis of tumor-infiltrating leukocytes
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
done one time, but CT26 and MethA tumor growth of SNS-ablated Mice were intraperitoneally injected with 6-OHDA hydrochloride
versus control mice was replicated at least three times for each cell (100 mg/kg; Sigma-Aldrich) on day −4 and 6-OHDA (200 mg/kg)
line. For tumor growth experiments, mice were excluded from anal- on days −2 and 0 to ablate sympathetic nerves. CT26 (5 × 105) cells
ysis if a palpable tumor was never detected, and all mice were eutha- were intradermally implanted into the right flank 4 hours after day 0
nized when average tumor diameter was measured to exceed 6-OHDA injection. Mice were euthanized either 6 or 18 days after
15 mm. Growth curves were analyzed up to the first day that a tumor challenge, and tumor-infiltrating leukocyte populations were
mouse in any group had a tumor that exceeded the 15-mm average analyzed by flow cytometry. Tumors were dissociated using the
diameter. Statistical analysis was performed as indicated in the fig- mouse Tumor Dissociation Kit (Miltenyi) according to the manu-
ure legends. Data are shown as means ± SEM for biological repli- facturer’s instructions, and the resulting suspension was analyzed
cates and means ± SD for technical replicates. by flow cytometry.
Mice Flow cytometry
Six- to 8-week-old female BALB/cJ mice were purchased from the Single-cell suspensions were stained using fluorochrome-labeled
Jackson laboratory. Mice were maintained in specific pathogen– antibodies against CD45.2 (104), CD11b (M1/70), Ly6C (HK1.4), Ly6G
free mouse facilities at the University of Connecticut Health Center. (1A8-Ly6g), CD101 (Moushi101), CXCR2 (SA044G4), S100A9 (2B10),
All experiments were approved and monitored by the University of CD3 (17A2), CD3 (145-2C11), CD4 (RM4-5), CD8a (53-6.7), CD62L
Connecticut Institutional Animal Care and Use Committee. (MEL-4), CD44 (IM7), CD25 (PC61.5), CD19 (eBio-1D3), FoxP3
(FJK-16s), or Ki67 (SolA15). All flow cytometry antibodies were
Chemical sympathectomy purchased from either BD Biosciences, eBioscience, or BioLegend.
Mice were intraperitoneally injected with 6-OHDA hydrochloride Either the Fixable Viability Dye eFluor780 (eBioscience) or the
(Sigma-Aldrich) to ablate sympathetic nerves. 6-OHDA was dis- Zombie UV Fixable Viability Kit (BioLegend) was used to exclude
solved in phosphate-buffered saline (PBS) with 0.1% ascorbic acid dead cells. For intracellular/intranuclear antigens, cells were fixed
(Sigma-Aldrich). Vehicle control–treated mice received intraperi- and permeabilized using the Foxp3/Transcription Factor Staining
toneal injections of PBS with 0.1% ascorbic acid. Buffer Set (eBioscience). Cell sorting was performed using the BD
FACSAria II Cell Sorter (BD Biosciences). Flow cytometry data
Tumor growth in SNS-ablated mice were collected using either BD LSR II (BD Biosciences) or MACSQuant
Mice were intraperitoneally injected with 6-OHDA hydrochloride (Miltenyi). Flow cytometry data were analyzed using FlowJo analy-
(100 mg/kg; Sigma-Aldrich) on day −4 and 6-OHDA (200 mg/kg) sis software (TreeStar).
on days −2 and 0 to ablate sympathetic nerves. CT26 colon carcinoma
(3.5 × 105), MethA fibrosarcoma (9.5 × 104), or MC38 colon carci- MDSC suppression assay
noma (1.25 × 105) cells were intradermally implanted into the right Wild-type BALB/c mice were intraperitoneally injected with 6-OHDA
flank 4 hours after day 0 6-OHDA injection. Subsequent injections hydrochloride (100 mg/kg; Sigma-Aldrich) on day −4 and 6-OHDA
of 6-OHDA (200 mg/kg) or vehicle control were administered (200 mg/kg) on days −2 and 0 to ablate sympathetic nerves. For
every 6 days for the duration of the experiment to maintain sympa- analysis of MDSC suppression in tumor-bearing mice, CT26 (3.5 ×
thetic denervation. Tumor growth was measured in two perpen- 105 to 5 × 105) cells were intradermally implanted into the right
dicular dimensions by caliper at least twice weekly. Tumor volume flank 4 hours after day 0 6-OHDA injection. Subsequent injections
was calculated using the formula V = (l × w2)/2. Mice were eutha- of 6-OHDA (200 mg/kg) or vehicle control were administered
nized when average tumor diameter reached ≥15 mm. CT26 every 6 days for the duration of the experiment to maintain sympa-
colon carcinoma cells were purchased from the American Type thetic denervation. Mice were euthanized 20 or 21 days after tumor
Culture Collection. MethA fibrosarcoma cells were originally ob- challenge. For analysis of MDSC suppression in non–tumor-bearing
tained from L. Old. mice, mice were euthanized 10 days after initiation of chemical de-
nervation. Spleens from five mice from each treatment group were pooled
Tumor growth in mice treated with AR antagonists and processed into a single-cell suspension. Red blood cells were lysed
Mice were intraperitoneally injected with propranolol hydrochloride using ACK (ammonium-chloride-potassium) (Thermo Fisher Scientific),
(10 mg/kg; Sigma-Aldrich), a combination of propranolol hydro- and CD11b+ cells were enriched using the EasySep Mouse CD11b
chloride (10 mg/kg) and phentolamine hydrochloride (10 mg/kg; Positive Selection Kit II (STEMCELL Technologies) according to the
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 9 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
manufacturer’s instructions. Viable, CD3−CD19−CD11b+ cells, CD3−CD19− In vivo S100A8/A9 quantification
CD11b+Ly6CintLy6G+ cells, CD3−CD19−CD11b+Ly6ChiLy6G− cells, or Blood was drawn from the facial vein of control-treated or SNS-
CD3−CD19−CD11b+Ly6C−Ly6G− cells were sorted from the resulting ablated wild-type BALB/c mice into Microtainer Gold tubes (BD
CD11b+-enriched splenocytes. T cells were isolated from the spleen of Biosciences) with SST (serum separation tube) gel and clot activa-
naïve, wild-type BALB/c mice using the EasySep Mouse CD4+ T Cell tor. Serum was isolated by centrifugation, and serum concentration
Isolation Kit (STEMCELL Technologies) or the EasySep Mouse CD8+ of S100A8/A9 heterodimer was determined using the Mouse S100A8/
T Cell Isolation Kit (STEMCELL Technologies). CD4+ or CD8+ T cells S100A9 Heterodimer DuoSet enzyme-linked immunosorbent assay
were labeled with 2.5 M carboxyfluorescein diacetate succinimidyl (ELISA) (R&D Biosystems). ELISA was performed according to the
ester (CFSE), cocultured with titrated numbers of sorted populations manufacturer’s instructions.
from SNS-ablated or control tumor-bearing mice, and activated using
Mouse T-Activator CD3/CD28 beads (Thermo Fisher Scientific). Single-cell RNA sequencing of non–T and non–B cells
T cell proliferation was evaluated by division index as calculated in from SNS-ablated tumor-bearing mice
FlowJo software. Wild-type BALB/c mice were intraperitoneally injected with
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
6-OHDA hydrochloride (100 mg/kg; Sigma-Aldrich) on day −4 and
In vitro MDSC generation 6-OHDA (200 mg/kg) on days −2 and 0 to ablate sympathetic
CT26 colon carcinoma cells were cultured in complete RPMI 1640 nerves. CT26 (5 × 105) cells were intradermally implanted into the
(Thermo Fisher Scientific) with 10% fetal bovine serum (FBS; Sigma- right flank 4 hours after day 0 6-OHDA injection. Subsequent injec-
Aldrich), 1 mM sodium pyruvate (Thermo Fisher Scientific), 1× tions of 6-OHDA (200 mg/kg) or vehicle control were administered
nonessential amino acids (Thermo Fisher Scientific), 1× penicillin/ every 6 days for the duration of the experiment to maintain sympa-
streptomycin/glutamine (Thermo Fisher Scientific), and 1× thetic denervation. Mice were euthanized 18 days after tumor chal-
-mercaptoethanol (Thermo Fisher Scientific) in a T75 flask until lenge, and spleen and bone marrow were harvested.
about 90% confluent. Supernatant (CT26-TCM), was then collected
and centrifuged for 5 min at 350g. Bone marrow was harvested from Single-cell labeling, capture, library preparation,
the femurs of a naïve BALB/c mouse, and 5 × 105 bone marrow cells and RNA sequencing
were cultured in 500 l of CT26-TCM supplemented with GM-CSF Single-cell suspensions from each sample were prepared from the
(20 ng/ml; PeproTech) and 100 M isoproterenol, clonidine, or spleen or bone marrow of SNS-ablated or control CT26-bearing
phenylephrine (Sigma-Aldrich) in a 24-well plate. After 3 days, culture wild-type BALB/c mice 18 days after tumor challenge. Viable,
was fed with 500 l of CT26-TCM with GM-CSF (20 ng/ml) and CD3−CD19− cells were sorted on a BD FACSAria II Cell Sorter
100 M AR agonist. Culture was harvested after 6 total days of culture, (BD Biosciences) into RPMI media containing 10% FBS. The
cells were counted on the Cellometer Auto 2000 Cell Viability Counter cells were washed and resuspended in PBS containing 0.04% bo-
(Nexcelom), and populations were assessed by flow cytometry. vine serum albumin and immediately processed as follows. Cells
were counted on a Countess II automated cell counter (Thermo
Gr-1+ cell depletion in SNS-ablated mice Fisher Scientific), and 12,000 cells were loaded into one lane of a
Gr-1+ cells were depleted from wild-type BALB/c mice by initial 10× Chromium microfluidic chip. Single-cell capture, barcoding,
intraperitoneal injection of 400 g of InVivoMAb anti-mouse Gr-1 and library preparation were performed using the 10× Chromium
(clone RB6-8C5) or isotype control (anti–keyhole limpet hemo- version 3 chemistry, according to the manufacturer’s protocol
cyanin, clone LTF-2) antibody (Bio X Cell) on day 0, followed by (no. CG000183). Complementary DNA and libraries were checked
6-OHDA (100 mg/kg) or vehicle control 4 hours later. Depletion for quality on an Agilent 4200 TapeStation, quantified by KAPA
was maintained by intraperitoneal injection of 200 g of anti–Gr-1 quantitative polymerase chain reaction, and sequenced on a sin-
antibody or isotype control on day 1, followed by 6-OHDA (200 mg/kg) gle lane of a HiSeq4000 (Illumina) to an average depth of 50,000
or vehicle control 4 hours later. Depletion was maintained again on reads per cell.
day 2 by intraperitoneal injection of 200 g of anti–Gr-1 antibody
or isotype control, and spleens were harvested and processed for Single-cell RNA sequencing analysis
flow cytometric analysis on day 3, 72 hours after initial anti–Gr-1 or CellRanger version 3.0.2 (10x Genomics) was used to process raw
isotype antibody administration. sequencing data and align sequencing reads to the mm10 genome.
Data analysis was performed using the SC1 single-cell sequencing
iTreg differentiation analysis tool (https://sc1.engr.uconn.edu/). Cells with greater than
Naïve CD4+ T cells (viable, CD4+, CD62Lhi, CD44low, and CD25−) 15% of reads mapping to mitochondrial genes or less than 500 de-
were sorted from the spleen of naïve wild-type BALB/c mice after tected genes were excluded from analysis. Clustering was performed
CD4+ T cell enrichment by negative selection using the EasySep using the TF-IDF (term frequency-inverse document frequency) clus-
Mouse CD4+ T Cell Isolation Kit (STEMCELL Technologies). T cells tering method and visualized using two- or three-dimensional t-SNE.
were activated in vitro using plate-bound CD3 antibody (1 g/ml) Differentially expressed genes in each cluster were defined by log2
and soluble CD28 antibody (1 g/ml). Cells were polarized in the fold change of ≥2 compared with expression in all other clusters.
presence of recombinant human TGF- (1.5 ng/ml; PeproTech) for
72 hours at 37°C. For experiments with S100A8/A9, iTregs were po- Calculation of RNA velocity of single cells
larized in the presence or absence of recombinant mouse S100A8/ RNA velocity for the single-cell data was calculated using the RNA
A9 heterodimer (10 g/ml; R&D Systems). In some experiments, velocity analysis workflow from La Manno et al. (31). The analysis
IL-2 was blocked using anti–IL-2 antibody (0.1 to 1 g/ml; JES6-5H4, model used the “constant velocity” assumption, and the RNA veloc-
BioLegend). ity estimates used pooling over 50 nearest neighbors.
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 10 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
Bulk RNA sequencing of tumor cell lines REFERENCES AND NOTES
mRNA from 1 × 106 CT26 colon carcinoma, MethA fibrosarcoma, 1. M. Hanoun, M. Maryanovich, A. Arnal-Estapé, P. S. Frenette, Neural regulation of
hematopoiesis, inflammation, and cancer. Neuron 86, 360–373 (2015).
or MC38 colon carcinoma cells was purified using a Qiagen AllPrep
2. M. Rosas-Ballina, K. J. Tracey, The neurology of the immune system: Neural reflexes
DNA/RNA mini kit. Transcriptome was sequenced using an Illumina regulate immunity. Neuron 64, 28–32 (2009).
sequencing platform, and gene expression estimation from RNA se- 3. F. Marino, M. Cosentino, Adrenergic modulation of immune cells: An update. Amino Acids
quencing data was performed by using the IsoEM2 algorithm (44). 45, 55–71 (2013).
Gene expression is reported as transcripts per million. 4. M. Rosas-Ballina, P. S. Olofsson, M. Ochani, S. I. Valdés-Ferrer, Y. A. Levine, C. Reardon,
M. W. Tusche, V. A. Pavlov, U. Andersson, S. Chavan, T. W. Mak, K. J. Tracey,
Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science
Time course of Treg expansion in SNS-ablated mice 334, 98–101 (2011).
Mice were intraperitoneally injected with 6-OHDA hydrochloride 5. S. B. Vasamsetti, J. Florentin, E. Coppin, L. C. A. Stiekema, K. H. Zheng, M. U. Nisar,
(100 mg/kg; Sigma-Aldrich) on day −1 and 6-OHDA (200 mg/kg) J. Sembrat, D. J. Levinthal, M. Rojas, E. S. G. Stroes, K. Kim, P. Dutta, Sympathetic neuronal
on day 0 to ablate sympathetic nerves. Mice were euthanized 1, 2, 4, activation triggers myeloid progenitor proliferation and differentiation. Immunity 49,
93–106.e7 (2018).
6, or 8 days after the completion of SNS ablation, spleens were har-
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
6. S. Moriyama, J. R. Brestoff, A.-L. Flamar, J. B. Moeller, C. S. N. Klose, L. C. Rankin,
vested, and Treg proportion and proliferation were determined by N. A. Yudanin, L. A. Monticelli, G. G. Putzel, H.-R. Rodewald, D. Artis, 2-adrenergic
flow cytometry. receptor–mediated negative regulation of group 2 innate lymphoid cell responses.
Science 359, 1056–1061 (2018).
Statistical analysis 7. L. P. Araujo, J. T. Maricato, M. G. Guereschi, M. C. Takenaka, V. M. Nascimento,
F. M. de Melo, F. J. Quintana, P. C. Brum, A. S. Basso, The sympathetic nervous system
Comparisons between two experimental conditions at a single time mitigates CNS autoimmunity via 2-adrenergic receptor signaling in immune cells.
point were performed by unpaired, two-tailed Student’s t test. Cell Rep. 28, 3120–3130.e5 (2019).
Comparisons between more than two experimental conditions 8. S. Bhowmick, A. Singh, R. A. Flavell, R. B. Clark, J. O’Rourke, R. E. Cone, The sympathetic
were performed by one- or two-way analysis of variance (ANOVA), nervous system modulates CD4+FoxP3+ regulatory T cells via a TGF--dependent
with either Tukey’s, Sidak’s, or Dunnett’s correction for multiple mechanism. J. Leukoc. Biol. 86, 1275–1283 (2009).
9. K. M. Grebe, K. Takeda, H. D. Hickman, A. L. Bailey, A. C. Embry, J. R. Bennink, J. W. Yewdell,
comparisons. Analysis of tumor growth curves was performed by Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and
two-way ANOVA. Analysis of survival upon tumor implantation exacerbates influenza A virus pathogenesis. J. Immunol. 184, 540–544 (2010).
was performed by log-rank (Mantel-Cox) test. Statistical signifi- 10. K. M. Grebe, H. D. Hickman, K. R. Irvine, K. Takeda, J. R. Bennink, J. W. Yewdell,
cance was reported as follows: *P < 0.05; **P < 0.01; ***P < 0.001; Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proc. Natl.
Acad. Sci. U.S.A. 106, 5300–5305 (2009).
****P < 0.0001. Specific statistical analysis for each experiment is
11. P. H. Thaker, L. Y. Han, A. A. Kamat, J. M. Arevalo, R. Takahashi, C. Lu, N. B. Jennings,
detailed in the figure legends. Comparisons between groups of mice G. Armaiz-Pena, J. A. Bankson, M. Ravoori, W. M. Merritt, Y. G. Lin, L. S. Mangala, T. J. Kim,
are considered to be biological replicates and are represented as R. L. Coleman, C. N. Landen, Y. Li, E. Felix, A. M. Sanguino, R. A. Newman, M. Lloyd,
means ± SEM. Comparisons between replicate wells of cell culture D. M. Gershenson, V. Kundra, G. Lopez-Berestein, S. K. Lutgendorf, S. W. Cole, A. K. Sood,
are considered to be technical replicates and are represented as Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian
carcinoma. Nat. Med. 12, 939–944 (2006).
means ± SD.
12. C. Magnon, S. J. Hall, J. Lin, X. Xue, L. Gerber, S. J. Freedland, P. S. Frenette, Autonomic nerve
development contributes to prostate cancer progression. Science 341, 1236361 (2013).
13. M. J. Bucsek, G. Qiao, C. R. MacDonald, T. Giridharan, L. Evans, B. Niedzwecki, H. Liu,
SUPPLEMENTARY MATERIALS K. M. Kokolus, J. W.-L. Eng, M. N. Messmer, K. Attwood, S. I. Abrams, B. L. Hylander,
immunology.sciencemag.org/cgi/content/full/5/51/eaay9368/DC1 E. A. Repasky, -Adrenergic signaling in mice housed at standard temperatures
Fig. S1. Ablation of SNS results in T cell–dependent increased rate of tumor growth in MethA suppresses an effector phenotype in CD8+T cells and undermines checkpoint inhibitor
fibrosarcoma and MC38 colon carcinoma tumor models. therapy. Cancer Res. 77, 5639–5651 (2017).
Fig. S2. CT26, MethA, and MC38 tumor cells lack ARs and transporters and do not respond to 14. L. Pedersen, M. Idorn, G. H. Olofsson, B. Lauenborg, I. Nookaew, R. H. Hansen,
adrenergic stimulation. H. H. Johannesen, J. C. Becker, K. S. Pedersen, C. Dethlefsen, J. Nielsen, J. Gehl,
Fig. S3. Representative MDSC gating strategy from SNS-ablated and control-treated B. K. Pedersen, P. T. Straten, P. Hojman, Voluntary running suppresses tumor growth
tumor-bearing mice. through epinephrine- and IL-6-Dependent NK cell mobilization and redistribution.
Fig. S4. Myeloid cells from SNS-ablated mice suppress CD4+ T cell proliferation. Cell Metab. 23, 554–562 (2016).
Fig. S5. Single-cell RNA sequencing analysis of CD3−CD19− splenocytes from SNS-ablated or 15. Y. Katayama, M. Battista, W.-M. Kao, A. Hidalgo, A. J. Peired, S. A. Thomas, P. S. Frentte,
control tumor-bearing mice. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress
Fig. S6. Identification of neutrophil-like cells in the spleen of CT26 tumor–bearing mice. from bone marrow. Cell 124, 407–421 (2006).
Fig. S7. Characterization of clusters in non-NK cells from tumor-bearing mice by analysis of 16. F. Veglia, M. Perego, D. Gabrilovich, Myeloid-derived suppressor cells coming of age.
selected differentially expressed genes. Nat. Immunol. 19, 108–119 (2018).
Fig. S8. Ablation of SNS alters splenic polymorphonuclear cells toward a more immature, 17. B. Huang, P.-Y. Pan, Q. Li, A. I. Sato, D. E. Levy, J. Bromberg, C. M. Divino, S.-H. Chen,
suppressive phenotype in tumor-bearing mice. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of
Fig. S9. Analysis of CD11b+Ly6CintLy6G+ cell maturity by flow cytometry validates findings of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 66,
single-cell RNA sequencing in spleen. 1123–1131 (2006).
Fig. S10. Single-cell RNA sequencing analysis of CD3−CD19− bone marrow cells from 18. P. Cheng, C. A. Corzo, N. Luetteke, B. Yu, S. Nagaraj, M. M. Bui, M. Ortiz, W. Nacken, C. Sorg,
SNS-ablated and control tumor-naïve mice. T. Vogl, J. Roth, D. I. Gabrilovich, Inhibition of dendritic cell differentiation and
Fig. S11. Analysis of neutrophil development by calculation of RNA velocity of single cells from accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9
the bone marrow. protein. J. Exp. Med. 205, 2235–2249 (2008).
Fig. S12. Gating strategy of MDSCs generated in vitro. 19. V. Bronte, D. B. Chappell, E. Apolloni, A. Cabrelle, M. Wang, P. Hwu, N. P. Restifo,
Fig. S13. Time course of SNS effect on Treg phenotype. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors
Fig. S14. Gating strategy and total numbers of iTregs generated in the presence of S100A8/A9. inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation.
Fig. S15. Efficiency of anti–Gr-1 antibody treatment in SNS-ablated or control mice. J. Immunol. 162, 5728–5737 (1999).
Fig. S16. Proposed model of SNS-mediated regulation of the accumulation of MDSCs and 20. C. M. Diaz-Montero, M. L. Salem, M. I. Nishimura, E. Garrett-Mayer, D. J. Cole, A. J. Montero,
tumor immunity. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer
Table S1. Raw data file. stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy.
View/request a protocol for this paper from Bio-protocol. Cancer Immunol. Immunother. 58, 49–59 (2009).
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 11 of 12
SCIENCE IMMUNOLOGY | RESEARCH ARTICLE
21. S. Solito, E. Falisi, C. M. Diaz-Montero, A. Doni, L. Pinton, A. Rosato, S. Francescato, 38. P. Martín, M. Gómez, A. Lamana, A. Cruz-Adalia, M. Ramírez-Huesca, M. A. Ursa,
G. Basso, P. Zanovello, G. Onicescu, E. Garrett-Mayer, A. J. Montero, V. Bronte, M. Yáñez-Mo, F. Sánchez-Madrid, CD69 association with Jak3/Stat5 proteins regulates
S. Mandruzzato, A human promyelocytic-like population is responsible for the immune Th17 cell differentiation. Mol. Cell. Biol. 30, 4877–4889 (2010).
suppression mediated by myeloid-derived suppressor cells. Blood 118, 2254–2265 (2011). 39. Y. Zhang, Q. Liu, M. Zhang, Y. Yu, X. Liu, X. Cao, Fas signal promotes lung cancer growth
22. S. Wang, R. Song, Z. Wang, Z. Jing, S. Wang, J. Ma, S100A8/A9 in inflammation. by recruiting myeloid-derived suppressor cells via cancer cell-derived PGE2. J. Immunol.
Front. Immunol. 9, 1298 (2018). 182, 3801–3808 (2009).
23. C.-R. Lin, T.-Y. W. Wei, H.-Y. Tsai, Y.-T. Wu, P.-Y. Wu, S.-T. Chen, Glycosylation-dependent 40. E. Schlecker, A. Stojanovic, C. Eisen, C. Quak, C. S. Falk, V. Umansky, A. Cerwenka,
interaction between CD69 and S100A8/S100A9 complex is required for regulatory T-cell Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate
differentiation. FASEB J. 29, 5006–5017 (2015). CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol.
24. H. Thoenen, J. P. Tranzer, The pharmacology of 6-hydroxydopamine. Annu. Rev. Pharmacol. 189, 5602–5611 (2012).
13, 169–180 (1973). 41. P.-Y. Pan, G. Ma, K. J. Weber, J. Ozao-Choy, G. Wang, B. Yin, C. M. Divino, S.-H. Chen,
25. V. Bronte, S. Brandau, S.-H. Chen, M. P. Colombo, A. B. Frey, T. F. Greten, S. Mandruzzato, Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory
P. J. Murray, A. Ochoa, S. Ostrand-Rosenberg, P. C. Rodriguez, A. Sica, V. Umansky, cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 70,
R. H. Vonderheide, D. I. Gabrilovich, Recommendations for myeloid-derived suppressor 99–108 (2010).
cell nomenclature and characterization standards. Nat. Commun. 7, 12150 (2016). 42. L. Yu, F. Yang, F. Zhang, D. Guo, L. Li, X. Wang, T. Liang, J. Wang, Z. Cai, H. Jin, CD69
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
26. F. Chalmin, S. Ladoire, G. Mignot, J. Vincent, M. Bruchard, J.-P. Remy-Martin, W. Boireau, enhances immunosuppressive function of regulatory T-cells and attenuates colitis by
A. Rouleau, B. Simon, D. Lanneau, A. De Thonel, G. Multhoff, A. Hamman, F. Martin, prompting IL-10 production. Cell Death Dis. 9, 905 (2018).
B. Chauffert, E. Solary, L. Zitvogel, C. Garrido, B. Ryffel, C. Borg, L. Apetoh, C. Rébé, 43. J. R. Cortés, R. Sánchez-Díaz, E. R. Bovolenta, O. Barreiro, S. Lasarte, A. Matesanz-Marín,
F. Ghiringhelli, Membrane-associated Hsp72 from tumor-derived exosomes mediates M. L. Toribio, F. Sánchez-Madrid, P. Martín, Maintenance of immune tolerance by
STAT3-dependent immunosuppressive function of mouse and human myeloid-derived Foxp3+ regulatory T cells requires CD69 expression. J. Autoimmun. 55, 51–62
suppressor cells. J. Clin. Invest. 120, 457–471 (2010). (2014).
27. C. A. Corzo, M. J. Cotter, P. Cheng, F. Cheng, S. Kusmartsev, E. Sotomayor, T. Padhya, 44. I. Mandric, Y. Temate-Tiagueu, T. Shcheglova, S. Al Seesi, A. Zelikovsky, I. I. Măndoiu,
T. V. McCaffrey, J. C. McCaffrey, D. I. Gabrilovich, Mechanism regulating reactive oxygen Fast bootstrapping-based estimation of confidence intervals of expression levels
species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701 and differential expression from RNA-Seq data. Bioinformatics 33, 3302–3304
(2009). (2017).
28. M. Evrard, I. W. H. Kwok, S. Z. Chong, K. W. W. Teng, E. Becht, J. Chen, J. L. Sieow, 45. M. Moussa, I. I. Măndoiu, Single cell RNA-seq data clustering using TF-IDF based methods.
H. L. Penny, G. C. Ching, S. Devi, J. M. Adrover, J. L. Y. Li, K. H. Liong, L. Tan, Z. Poon, S. Foo, BMC Genomics 19, 569 (2018).
J. W. Chua, I.-H. Su, K. Balabanian, F. Bachelerie, S. K. Biswas, A. Larbi, W. Y. K. Hwang,
V. Madan, H. P. Koeffler, S. C. Wong, E. W. Newell, A. Hidalgo, F. Ginhoux, L. G. Ng, Acknowledgments: We thank A. Hagymasi and T. Shcheglova from the Carole and Ray Neag
Developmental analysis of bone marrow neutrophils reveals populations specialized Comprehensive Cancer Center at UConn Health for bulk RNA sequencing and analysis of
in expansion, trafficking, and effector functions. Immunity 48, 364–379.e8 (2018). tumor cell lines. We thank E. Jellison and L. Zhu at the UConn Health Flow Cytometry Core for
29. Y. P. Zhu, L. Padgett, H. Q. Dinh, P. Marcovecchio, A. Blatchley, R. Wu, E. Ehinger, C. Kim, assistance with fluorescence-activated cell sorting and D. Ruan and M. Samuels at the
Z. Mikulski, G. Seumois, A. Madrigal, P. Vijayanand, C. C. Hedrick, Identification of an early JAX-UConn Single Cell Genomics Center for 10× single-cell library preparation and RNA
unipotent neutrophil progenitor with pro-tumoral activity in mouse and human bone sequencing. Funding: This work was supported by the Neag Cancer Immunology Translation
marrow. Cell Rep. 24, 2329–2341.e8 (2018). Fund at the Neag Cancer Center. Author contributions: J.T.N. and P.K.S. designed experiments.
30. G. La Manno, R. Soldatov, A. Zeisel, E. Braun, H. Hochgerner, V. Petukhov, K. Lidschreiber, J.T.N. and W.L.C. performed experiments. M.M. and I.I.M. designed and developed analytical
M. E. Kastriti, P. Lönnerberg, A. Furlan, J. Fan, L. E. Borm, Z. Liu, D. van Bruggen, J. Guo, tools. M.M., I.I.M., and J.T.N. performed computational analysis. J.T.N. and P.K.S. wrote the
X. He, R. Barker, E. Sundström, G. Castelo-Branco, P. Cramer, I. Adameyko, S. Linnarsson, paper. P.K.S. supervised the project. Competing interests: I.I.M. is a cofounder and holds an
P. V. Kharchenko, RNA velocity of single cells. Nature 560, 494–498 (2018). interest in SmplBio LLC, a company developing cloud-based single-cell RNA sequencing
31. H. Alshetaiwi, N. Pervolarakis, L. L. McIntyre, D. Ma, Q. Nguyen, J. A. Rath, K. Nee, analysis software. No products, services, or technologies of SmplBio have been evaluated or
G. Hernandez, K. Evans, L. Torosian, A. Silva, C. Walsh, K. Kessenbrock, Defining the tested in this work. Data and materials availability: The processed single-cell RNA
emergence of myeloid-derived suppressor cells in breast cancer using single-cell sequencing data used in this paper and RNA sequencing data of tumor cell lines are
transcriptomics. Sci. Immunol. 5, eaay6017 (2020). publicly accessible at https://urldefense.com/v3/__https://dna.engr.uconn.edu/?page_
32. C. Godinho-Silva, F. Cardoso, H. Veiga-Fernandes, Neuro–immune cell units: A new id=971__;!!N0rdg9Wr!8dU8gl0FDnxHmU_JPS3UGl2pxi1XnTYWrprKwqKkrqsqJo8E5FqLsc48_
paradigm in physiology. Annu. Rev. Immunol. 37, 19–46 (2019). F2RTuLMaA$. The data are in the process of deposition to GEO; as a part of that submission,
33. B. P. Ramos, A. F. T. Arnsten, Adrenergic pharmacology and cognition: Focus on the they have been submitted to the Sequence Read Archive from NCBI under the submission
prefrontal cortex. Pharmacol. Ther. 113, 523–536 (2007). number SUB7800971, title: “Sympathetic nervous tone limits the development of
34. H. Mohammadpour, C. R. MacDonald, G. Qiao, M. Chen, B. Dong, B. L. Hylander, myeloid-derived suppressor cells.” The submission status shows the following: BioProject:
P. L. McCarthy, S. I. Abrams, E. A. Repasky, 2 Adrenergic receptor–mediated signaling Processed and successfully loaded:PRJNA647841.
regulates the immunosuppressive potential of myeloid-derived suppressor cells.
J. Clin. Invest. 129, 5537–5552 (2019). Submitted 30 July 2019
35. C. Kerkhoff, H. A. Hofmann, J. Vormoor, H. Melkonyan, J. Roth, C. Sorg, M. Klempt, Binding Accepted 29 July 2020
of two nuclear complexes to a novel regulatory element within the human S100A9 Published 11 September 2020
promoter drives the S100A9 gene expression. J. Biol. Chem. 277, 41879–41887 (2002). 10.1126/sciimmunol.aay9368
36. J. B. Wing, A. Tanaka, S. Sakaguchi, Human FOXP3+ regulatory T cell heterogeneity
and function in autoimmunity and cancer. Immunity 50, 302–316 (2019). Citation: J. T. Nevin, M. Moussa, W. L. Corwin, I. I. Mandoiu, P. K. Srivastava, Sympathetic
37. S. Nagaraj, J.-I. Youn, D. I. Gabrilovich, Reciprocal relationship between myeloid-derived nervous tone limits the development of myeloid-derived suppressor cells. Sci. Immunol. 5,
suppressor cells and T cells. J. Immunol. 191, 17–23 (2013). eaay9368 (2020).
Nevin et al., Sci. Immunol. 5, eaay9368 (2020) 11 September 2020 12 of 12
Sympathetic nervous tone limits the development of myeloid-derived suppressor cells
James T. Nevin, Marmar Moussa, William L. Corwin, Ion I. Mandoiu and Pramod K. Srivastava
Sci. Immunol. 5, eaay9368.
DOI: 10.1126/sciimmunol.aay9368
Downloaded from http://immunology.sciencemag.org/ at CORNELL UNIVERSITY on September 15, 2020
A sympathetic tumor response
The sympathetic nervous system (SNS) has been shown to regulate immune responses through various
mechanisms. Nevin et al. now show that ablation of SNS signaling can suppress tumor immunity, and this is caused by
disruption in α-adrenergic signaling that is needed for myeloid cell maturation. In tumor-bearing mice, this disruption
promotes the accumulation of immature myeloid-derived suppressor cells, which allows for tumor growth. In the absence
of intact SNS signaling, MDSCs also promote expansion of regulatory T cells by secreting the alarmin heterodimer
S100A8/A9. These results provide insight into the contributions of SNS signaling in innate and adaptive immunity,
particularly in the context of tumor immunity.
ARTICLE TOOLS http://immunology.sciencemag.org/content/5/51/eaay9368
SUPPLEMENTARY http://immunology.sciencemag.org/content/suppl/2020/09/04/5.51.eaay9368.DC1
MATERIALS
REFERENCES This article cites 45 articles, 18 of which you can access for free
http://immunology.sciencemag.org/content/5/51/eaay9368#BIBL
Use of this article is subject to the Terms of Service
Science Immunology (ISSN 2470-9468) is published by the American Association for the Advancement of Science, 1200
New York Avenue NW, Washington, DC 20005. The title Science Immunology is a registered trademark of AAAS.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of
Science. No claim to original U.S. Government Works
You might also like
- Danchin 2008Document10 pagesDanchin 2008Sol LakosNo ratings yet
- 10 1007@BF02191557Document10 pages10 1007@BF02191557Sol LakosNo ratings yet
- Selvarajah 2013Document7 pagesSelvarajah 2013Sol LakosNo ratings yet
- Pelletier 2014Document8 pagesPelletier 2014Sol LakosNo ratings yet
- Labinaz 2019Document7 pagesLabinaz 2019Sol LakosNo ratings yet
- Oberlander 2016Document14 pagesOberlander 2016Sol LakosNo ratings yet
- Westenbroek 2004Document6 pagesWestenbroek 2004Sol LakosNo ratings yet
- 1 s2.0 S0140673620326581 MainDocument14 pages1 s2.0 S0140673620326581 MainSol LakosNo ratings yet
- BMJ Insuficiencia Mitral y Estenosis Ao en EmbarazoDocument4 pagesBMJ Insuficiencia Mitral y Estenosis Ao en EmbarazoSol LakosNo ratings yet
- Lancet 2016 Valvulopatias Estenosis AórticaDocument12 pagesLancet 2016 Valvulopatias Estenosis AórticaSol LakosNo ratings yet
- Seminar: EpidemiologyDocument13 pagesSeminar: EpidemiologySol LakosNo ratings yet
- Sciimmunol Abe9950Document18 pagesSciimmunol Abe9950Sol LakosNo ratings yet
- The Spread of MisinformationDocument2 pagesThe Spread of MisinformationSol LakosNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Chapter-11 Respiration in Plants PDFDocument26 pagesChapter-11 Respiration in Plants PDFVedavathi100% (1)
- Artepillin C As An Outstanding Phenolic Compound of Brazilian Green Propolis For Disease Treatment: A Review On Pharmacological AspectsDocument13 pagesArtepillin C As An Outstanding Phenolic Compound of Brazilian Green Propolis For Disease Treatment: A Review On Pharmacological AspectslyviaNo ratings yet
- ECB3e Chapter 20Document80 pagesECB3e Chapter 20Sarifa Ghina RaquelleNo ratings yet
- Protein Synthesis Model LabDocument7 pagesProtein Synthesis Model Labmarisa corderoNo ratings yet
- Target Therapies in Pancreatic CancerDocument6 pagesTarget Therapies in Pancreatic CancerReneNo ratings yet
- CLS 715 Immunological Synapse 2023Document14 pagesCLS 715 Immunological Synapse 2023Heba LahhamNo ratings yet
- Biological Macromolecules: Nucleic Acids Proteins Carbohydrates LipidsDocument1 pageBiological Macromolecules: Nucleic Acids Proteins Carbohydrates LipidsJoan PamatNo ratings yet
- Eoc Review Packet 2018Document25 pagesEoc Review Packet 2018api-375285021No ratings yet
- PharmacodynamicsDocument19 pagesPharmacodynamicsBin HipNo ratings yet
- HHS Public Access: Brain Photobiomodulation Therapy: A Narrative ReviewDocument57 pagesHHS Public Access: Brain Photobiomodulation Therapy: A Narrative ReviewarexixNo ratings yet
- Introduction To HematologyDocument16 pagesIntroduction To HematologyAsxe CeeNo ratings yet
- PAS I Exercise Discussion Compressed 231124 171104Document156 pagesPAS I Exercise Discussion Compressed 231124 171104Suryadi SuryadiNo ratings yet
- CIE IGCSE Biology 17 RNDocument22 pagesCIE IGCSE Biology 17 RNfareezsunshinemirzaNo ratings yet
- Cell Disruption Extracting Target Product: Location of Biological ProductsDocument88 pagesCell Disruption Extracting Target Product: Location of Biological ProductsRoshan jaiswalNo ratings yet
- 10.1 Cell Growth, Division and ReproductionDocument6 pages10.1 Cell Growth, Division and ReproductionJJNo ratings yet
- Biomolecules: A. Synthesis & Hydrolysis Reaction VocabDocument18 pagesBiomolecules: A. Synthesis & Hydrolysis Reaction Vocabrashmi_harryNo ratings yet
- Chymosin ReninDocument1 pageChymosin ReninArfanizam Bin ArisNo ratings yet
- 18 Smooth MuscleDocument3 pages18 Smooth Muscleمحمود محمدNo ratings yet
- Lehninger Principles of Biochemistry Sixth EditionDocument6 pagesLehninger Principles of Biochemistry Sixth Editiontest bank for you0% (2)
- Answer: Activation of Jaks of Cytokine-Receptor Cytoplasmic Domains inDocument2 pagesAnswer: Activation of Jaks of Cytokine-Receptor Cytoplasmic Domains inSalekin MishuNo ratings yet
- XI Botany ChapterWise Prvs Questions HssliveDocument25 pagesXI Botany ChapterWise Prvs Questions Hsslivejithu63% (8)
- Cell Division: Mitosis and MeiosisDocument38 pagesCell Division: Mitosis and MeiosisMaika Ysabelle RavaloNo ratings yet
- Melaka BoosterDocument12 pagesMelaka BoosterHaifa amirahNo ratings yet
- DURAGEN Plus Dural Regeneration MatrixDocument8 pagesDURAGEN Plus Dural Regeneration MatrixramadhaniregulatoryNo ratings yet
- Provincial Exam Multiple Choice Question Guide 2000Document19 pagesProvincial Exam Multiple Choice Question Guide 2000Hansoo YoonNo ratings yet
- Subject: Biochemistry Topic:Lipid Metabolism 2 Lecturer: Dr. Laygo DATE: NOV. 2010Document11 pagesSubject: Biochemistry Topic:Lipid Metabolism 2 Lecturer: Dr. Laygo DATE: NOV. 2010Std DlshsiNo ratings yet
- Assignment 1 Biol 1700 Winter 2021 FinalDocument13 pagesAssignment 1 Biol 1700 Winter 2021 Finalapi-546520104No ratings yet
- Telomere-Related Markers For Cancer: Xiaotian Yuan, Mingkai Dai and Dawei XuDocument23 pagesTelomere-Related Markers For Cancer: Xiaotian Yuan, Mingkai Dai and Dawei XuathayafebNo ratings yet
- The Immune SystemDocument19 pagesThe Immune Systemmay esteban100% (2)
- Morals and Ethics of Using Stem CellsDocument2 pagesMorals and Ethics of Using Stem Cellssfe sfgNo ratings yet