Professional Documents
Culture Documents
Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis - ClinicalKey
Uploaded by
MasithaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis - ClinicalKey
Uploaded by
MasithaCopyright:
Available Formats
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.
15
CLINICAL OVERVIEW
Stevens-Johnson syndrome and toxic epidermal
necrolysis
Elsevier Point of Care (see details)
Updated 2 Februari 2021. Copyright Elsevier BV. All rights reserved.
Synopsis
Key Points Urgent Action
Stevens-Johnson syndrome and toxic epidermal
Consult appropriate organ
necrolysis are acute mucocutaneous reactions that differ
system specialist to treat
by severity
complications
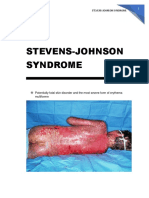
In Stevens-Johnson syndrome, extensive blisters can
lead to skin detachment affecting less than 10% of Monitor for secondary
BSA; in toxic epidermal necrolysis, more than 30% of infections and sepsis
BSA is affected; intermediate levels represent an
Monitor prognosis parameters
overlap of the entities 1
Initial symptoms may resemble a viral syndrome with
rash, but in a few days, skin and mucosal eruption lead to rapid deterioration of clinical
condition, and ICU or burn unit admission is required
Most often Stevens-Johnson syndrome or toxic epidermal necrolysis is caused by drug
therapy; however, Mycoplasma pneumoniae and HSV have been identified in cases without
drug exposure
Punch biopsy analysis confirms diagnosis, showing necrotic epidermis and effects that involve
all layers of skin
Erythema multiforme, staphylococcal scalded skin syndrome, autoimmune blister disorders,
and drug reaction with eosinophilia and systemic symptoms need to be ruled out before the
diagnosis of Stevens-Johnson syndrome or toxic epidermal necrolysis can be made
Treatment goals are to protect skin from irritations and infections, to control pain using
medications, and to provide electrolytes and nutrition as needed in supportive care
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 1 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Complications may arise in multiple organ systems, including skin and mucosal surfaces
(especially genital), eyes, respiratory system, and gastrointestinal tract
Pitfalls
Avoid misdiagnosis of other cutaneous eruptions as Stevens-Johnson syndrome or toxic
epidermal necrolysis
These conditions are rare (on the order of a handful of cases per million person-years) 2
Terminology
Clinical Clarification
Stevens-Johnson syndrome and toxic epidermal necrolysis are rare, life-threatening skin
conditions involving blistering, skin loss, and multiorgan damage; they are usually caused by
drug therapy
Classification
Stevens-Johnson syndrome exists at the less severe end of a clinical spectrum of acute
mucocutaneous reactions; toxic epidermal necrolysis represents a higher level of severity 3
Severity is defined primarily by extent of blistering 1
In Stevens-Johnson syndrome, extensive blisters lead to skin detachment affecting less than
10% of BSA
In toxic epidermal necrolysis, eruption and scalding lead to skin detachment affecting more
than 30% of BSA
Stevens-Johnson syndrome and toxic epidermal necrolysis may overlap with skin
detachment at 10% to 30% of BSA
Diagnosis
Clinical Presentation
History
Initial symptoms may be nonspecific 2
Fever, conjunctival irritation, rhinitis, cough, sore
throat, and malaise
Areas of painful skin and/or mucosa may occur in the
absence of visible changes
Within several days, skin and mucus membrane lesions
appear
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 2 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
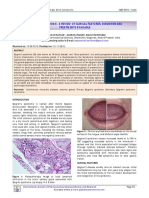
First appear on face and trunk, spreading quickly to Stevens-Johnson syndrome. - Large
rest of body, including mucosae of eyes, nose, desquamation in Stevens-Johnson
oropharynx, and perineum syndrome.
Mucositis and pain at mouth, lips, and tongue may
limit oral intake
Involvement of gastrointestinal mucosa may result in Ocular surface involvement in acute
abdominal distention and sometimes diarrhea, which Stevens-Johnson syndrome/toxic
epidermal necrolysis. - A, Conjunctival
may be bloody 4
hyperemia and membrane. B, Eyelid
Involvement of perineum and urinary tract may lead margin sloughing (arrow) as evident
to dysuria, hematuria, or urinary retention 4 with fluorescein staining under cobalt
blue light. C, Corneal epithelial defect
Involvement of respiratory tract may cause cough, (arrow) stained with fluorescein.
dyspnea, or hemoptysis 4
Medication history may provide important clues to cause
2
More than 100 drugs have been implicated; the
frequency with which a drug has been implicated is
important in determining its potential role, especially
in patients taking multiple medications
Timing of start of drug administration is also an Stevens-Johnson syndrome
important factor in determining causality; drug- associated with afatinib treatment. - A
induced cases usually begin within 8 weeks of start of and B, before treatment. C and D,
the inciting agent, and most often within 4 weeks (eg, after 2 weeks of treatment. Cutaneous
5
a mean of 15 days in one study) lesions improve slowly.
Physical examination
Findings depend on stage at which patient is seen, as
extent and nature of lesions evolve
Inspection of skin and mucosae to determine
morphology and extent of lesions and characteristics of
erosion 6
Lesions generally originate on face and trunk, then Typical crusted lips in a patient with
form peripherally; extremities are less affected than Stevens-Johnson syndrome.
face and trunk, although palms and soles may be
involved
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 3 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Lesions begin as erythematous macules that enlarge, and they often have a target-like
appearance; the lesions coalesce and develop flaccid blisters that may break, oozing serous
or bloody fluid
Positive Nikolsky sign is suggestive but not confirmatory 6
Tangential mechanical pressure on intact lesion induces epidermal detachment
Oral lesions and purulent conjunctivitis are usually present
Perineal mucosa is typically involved
Assessment of general health and status of internal organs
Vital signs
Fever is a common feature and does not necessarily imply presence of infection
Elevation of pulse and respiratory rate, and decrease in blood pressure, may occur with
superimposed infections or sepsis
Sloughing of respiratory mucosa may impair respiration; cough, wheezing, or rhonchi may
be audible
Causes and Risk Factors
Causes
Medications are the usual causative agents (75% 7-85% 1 of cases)
High-risk drugs 6 8
Allopurinol is the most common cause in Europe and Israel; risk is increased with
coadministration of an ACE inhibitor 2
Antibiotics are among the most common causes 9
Trimethoprim-sulfamethoxazole and other sulfonamide antibiotics 2
Aminopenicillins 10
Antituberculosis drugs 10
Anticonvulsants
Carbamazepine
Phenytoin
Phenobarbital
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 4 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Lamotrigine (especially in conjunction with valproic acid) 2
NSAIDs of the oxicam class (eg, meloxicam, piroxicam, tenoxicam)
Nevirapine 2
Biologic agents 8
Reexposure to previously implicated medications may result in a recurrence
Other causes include infections
Mycoplasma pneumoniae
HSV
HIV
Annual incidence of toxic epidermal necrolysis is 1000-fold higher in patients with HIV
than in the general population 6
20% of cases are idiopathic 2
Risk factors and/or associations
Age
Stevens-Johnson syndrome and toxic epidermal necrolysis can occur at any age, with higher
prevalence in adults 11
Sex
Predominance in women versus men was reported in the past 7 11
With HIV epidemic, current incidence is approximately the same in men and women 7
Genetics
Increased risk of hypersensitivity reaction to specific drugs is linked to certain HLA antigens 8
12
Numerous HLA alleles have been associated with this disease, and some have higher
prevalence in people with Asian ancestry
Lamotrigine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis are
associated with presence of HLA-B*15:02 allele in people with Han Chinese ancestry 13
Increased risk of phenytoin-induced Stevens-Johnson syndrome and toxic epidermal
necrolysis occurs with HLA-B*15:02 allele, which is inherited mostly via Asian ancestry and
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 5 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
not as much via European ancestry or African ancestry 10 14
HLA-B*58:01 is associated with allopurinol-induced severe cutaneous adverse reactions 15
16
HLA-B*15:02 is associated with phenytoin-induced and carbamazepine-induced Stevens-
Johnson syndrome and toxic epidermal necrolysis 14
HLA-B12 is associated with a higher risk for toxic epidermal necrolysis 9
Ethnicity/race
HLA-B*15:02 allele is inherited mostly via Asian ancestry and not as much via European
ancestry or African ancestry 13 14
Other risk factors/associations
Systemic lupus erythematosus has been reported as a predisposing condition
Some authorities distinguish the blistering skin condition associated with this disease from
Stevens-Johnson syndrome and toxic epidermal necrolysis, attributing it to a different
process 10
Bone marrow transplant recipients are at increased risk for the disease 9
Presence of malignancy or collagen vascular disease increases risk 17
Diagnostic Procedures
Primary diagnostic tools
Diagnosis is often based on details from history and physical examination
Skin biopsy is recommended to confirm diagnosis and rule out differential diagnoses 1
Because of possible complications due to fluid losses, systemic involvement, and
superimposed infection, comprehensive laboratory evaluation is warranted and should be
based on clinical circumstances and degree of illness
CBC, electrolyte levels, serum or plasma glucose level, renal function tests (eg, BUN and
creatinine levels), liver function tests, coagulation studies, and arterial blood gas levels
are recommended in all patients 1 4 10
Elements of a basic metabolic profile are factored into the SCORTEN score, a validated
tool for assessing severity 10 18
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 6 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Obtain serology or skin or ocular swabs for the following infections if clinical suspicion
exists: 4
Mycoplasma pneumoniae
HSV
HIV
Chlamydia
Varicella-zoster virus
Adenovirus
HLA testing for alleles known to predispose to the disease may help to identify a possible
cause 10
Associations are quite specific; certain alleles predispose to reactions to specific drugs and
are more prevalent via Asian ancestry than via other ancestries
Testing should be guided by an expert
Laboratory
Functional testing
Procedures
Differential Diagnosis
Acute generalized exanthematous pustulosis. - Edematous and erythematous pustular eruption on the back.
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 7 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Bullous pemphigoid. - Tense bullae on an erythematous urticarial base of skin.
Burns: depth of thermal injury. - A, Patient with sunburn on lower extremity (a superficial or first-degree burn
with associated blisters on anterior tibial surface). B, Partial-thickness injury of hand (superficial second-
degree burn). C, Partial-thickness injury extending beyond subcutaneous layers (deep second-degree burn).
D, Full-thickness (third-degree) burn. E, Full-thickness injury with extensive tissue loss (fourth-degree burn).
Erythema multiforme with typical target lesions.
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 8 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Staphylococcal scalded skin syndrome. - Blisters are expression of toxin-related (exfoliative toxin A or B)
distant disease and usually do not contain microorganisms.
General
Rarity of Stevens-Johnson syndrome and toxic epidermal necrolysis informs the effort to avoid
misdiagnosis of other cutaneous eruptions
Stevens-Johnson syndrome: 1 to 6 cases per million person-years 2
Toxic epidermal necrolysis: 0.4 to 1.2 cases per million person-years 2
Most common
Erythema multiforme 20
Formerly sometimes equated with Stevens-Johnson syndrome, but now distinguished from it
Presents as target lesions having 3 or more concentric rings in a symmetrical distribution with or
without blisters
Nikolsky sign is not present in erythema multiforme major (ie, no exfoliation of outermost layer
of skin just from slight rubbing)
Differentiated histologically by mononuclear cell infiltration and less necrosis than in Stevens-
Johnson syndrome
Acute generalized exanthematous pustulosis 20
Presents as widespread erythematous and edematous rash with fever and abrupt onset
Distinguished by small nonfollicular whitish pustules that disappear spontaneously in a couple
of days
Differentiated histologically by migration of PMNs within epidermis and paucity of necrotic
keratinocytes
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 9 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Drug reaction with eosinophilia and systemic symptoms (drug-induced hypersensitivity
syndrome) 21
Like Stevens-Johnson syndrome and toxic epidermal necrolysis, it is a severe cutaneous adverse
reaction occurring usually several weeks after introduction of causative drug
Presents as a pruriginous maculopapular rash (usually starting on face and then generalized),
with edema of face and extremities, fever, and peripheral lymphadenopathy; mucosal
involvement is unusual
Systemic involvement, particularly in liver and kidneys, is nearly always present
Laboratory tests find high eosinophil count, atypical lymphocytes, and sometimes elevated liver
enzyme levels and/or decreased GFR
Differentiated by pattern of dermatologic features, high eosinophil count, and atypical
lymphocytes
Second-degree burn (Related: Thermal burns)
Presents as partial-thickness loss of skin from heat injury
Distinguished by localization of lesions and complete absence of lesions in unaffected areas
Differentiated by history and size of lesions (often quite large)
Staphylococcal scalded skin syndrome
Clinical presentation is blistering skin lesions in an infant
Nikolsky sign is positive, as in Stevens-Johnson syndrome and toxic epidermal necrolysis
Differentiated histologically by subcorneal split with or without mild acantholysis (loss of cell to
cell adhesion)
Autoimmune bullous disorders 202223
Present as blisters of variable character (eg, papular, urticariform, pruritic)
Distinguished by autoantibodies arising in epidermis, dermal-epidermal junction, and basement
membrane
Differentiated histologically by linear deposition of immunoglobulin (IgG and C3, identified by
fluorescent antibodies) at basement membrane
Treatment
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 10 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Goals
Discontinue or remove inciting agent, if identified
Protect skin and provide supportive care to promote healing
Avoid infections and complications
Disposition
Admission criteria
Hospitalize all patients with confirmed Stevens-Johnson syndrome or toxic epidermal necrolysis
19
Criteria for ICU admission
Patients with SCORTEN score of 3 or higher should be managed in ICU or burn unit 6
Recommendations for specialist referral
Consult dermatologist for specialized biopsy and skin treatment techniques
Consult ophthalmologist to assess and treat ocular involvement
Additional specialists may be needed as clinical status dictates
Treatment Options
Treatment is supportive and similar to that for major burns
Guidelines are available, but evidence base is limited 1 4 19 24
NIH recommendation to begin standardized case reporting reflects an effort to collect more
data 25
Discontinue any possible offending agents and nonessential medications promptly 10
General principles of treatment for all patients in ICU setting
Meticulous wound care 10
Debridement of detached epidermis is not recommended; blisters should be decompressed
4
Regularly clean wounds and intact skin with warm sterile water, saline, or chlorhexidine 4
Apply a greasy emollient all over skin, including lesions, during acute phase; use of an
aerosolized form may limit epidermal trauma 4
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 11 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Apply nonadhesive dressings to wounds and uninvolved skin (eg, pressure points, friction
sites) 4
Fluid replacement to maintain urine output of 0.5 to 1 mL/kg/hour 10
Crystalloids are recommended; potassium supplementation is often needed 6
Supplemental albumen is recommended by some authorities 10
Pain control 10
May require opioids if pain is severe
Nutritional support
Enteral is preferred (ie, liquid or soft diet) 10
Ventilatory support if needed
Ambient temperature should be maintained at 28 °C to 32 °C 10
Antihistamines may be administered for pruritus from Stevens-Johnson syndrome
Very high potency topical corticosteroids may be applied to affected areas of noneroded skin
once infection has been excluded 4
Mouth care for patients with involvement of oropharyngeal mucosa
Antiseptic mouthwash is recommended
Antiinflammatory mouthwash or oral spray may be used, particularly before eating 4
Potent topical corticosteroid mouthwash may be used 4
Eye care for patients with ocular involvement 4
Daily ophthalmology review
Daily saline irrigation
Ocular lubricants and dressings to prevent corneal exposure
Consider topical corticosteroid eye drops, provided infection has been excluded
Consider amniotic membrane transplantation for conjunctival epithelial defects
Consider topical antibiotic prophylaxis for corneal erosion or ulceration
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 12 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Consider immunomodulatory therapy 26
Commonly used, but evidence of benefit is limited and use is controversial 26
In a small case series, IV immunoglobulin appeared to improve survival, especially at doses of
2 g/kg or higher; however, large studies have found no survival advantage 27 28
Systemic corticosteroids are frequently used, but studies have not consistently found benefit;
29 consider use in combination with IV immunoglobulin or in combination with cyclosporine
30 31 32
Several other systemic therapies (eg, cyclosporine, tumor necrosis factor antagonists) have
been used, but there is no clear drug or regimen of choice 6 10 29 33
Ideally start within first 24 to 48 hours after presentation if used 32
Surgical debridement is indicated for necrotic or infected epidermis 4
Comorbidities that may have a role in the outbreak (eg, infections) should be addressed (eg,
acyclovir for HSV infection)
Drug therapy
IV immunoglobulin
Immune Globulin (Human) Solution for injection; Infants, Children, and Adolescents:
Efficacy data are limited and variable; reported dosage regimens vary. Total dose of 2 g/kg
IV divided over 1 to 4 days has been used. Reported dosage range: 1.5 to 5.8 g/kg IV total
dose (given in divided doses).
Immune Globulin (Human) Solution for injection; Adults: Total dose of 2 to 3 g/kg IV
divided over 3 to 4 days has been used. 6
Dexamethasone
For Stevens-Johnson syndrome:
Dexamethasone Sodium Phosphate Solution for injection; Adolescents, Children, and
Infants: 0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided
doses is the FDA-approved general dosage range. Adjust according to patient response.
Dexamethasone Sodium Phosphate Solution for injection; Adults: Initially, 0.5 to 9
mg/day IV or IM, in 2 to 4 divided doses. Adjust according to patient response.
For toxic epidermal necrolysis:
Dexamethasone Sodium Phosphate Solution for injection; Adults: 1.5 mg/kg IV given
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 13 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
over 30 to 60 minutes daily for 3 days. 10
Methylprednisolone 26 31
Pulse therapy
Methylprednisolone Sodium Succinate Solution for injection; Adults, Adolescents and
Children: 250 to 1,000 mg IV given once daily or on alternate days for 3 to 5 doses. 31
Cyclosporine 26
Cyclosporine Oral capsule; Adults, Adolescents, and Children: 3 to 5 mg/kg/day PO in
divided doses for up to 21 days has been recommended. The average duration is 7 days; may
be given intravenously if needed. 34
Nondrug and supportive care
Limit skin trauma by avoidance or careful use of medical items such as blood pressure cuffs,
tourniquets, ECG electrodes, and dressings 4
Cover areas of desquamation with nonadherent dressings; also apply to noninvolved sites if
protection is required (eg, friction areas) 4
Dressings may be biologic or biosynthetic 1
Dressings may be silver- or antibiotic-impregnated
Frequent changes are not recommended
Encourage mobilization under supervision of physical therapist 4
Comorbidities
Comorbidities that may have a role in the outbreak (eg, infections) should be addressed (eg,
acyclovir for HSV infection)
Special populations
Children
Mortality rate is lower in children 35
Half of affected children have long-term complications 35
A guideline focused on children and adolescents is available from the British Association of
Dermatologists 4
Older adults (eg, those older than 60 years)
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 14 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
In adults older than 60 years, underlying diseases may contribute to mortality 36
Monitoring
Monitor for secondary infections and sepsis
Monitor prognosis parameters
Complications and Prognosis
Complications
Secondary infections
Sepsis
Permanent skin damage 35
Hypopigmentation or hyperpigmentation 37
Scarring of skin
Photosensitivity 37
Chronic pruritis 37
Ocular complications
Keratitis
Corneal defects
Chronic conjunctivitis
Vision loss
Severe dry eye 37
Phimosis or urethral strictures (Related: Phimosis and paraphimosis)
Vaginal adhesions, stenosis, dryness, and dyspareunia 37
Sclerosing cholangitis
Bronchiolitis obliterans
Stridor
Recurrence of Stevens-Johnson syndrome
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 15 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Death
Prognosis
Prognosis is correlated with SCORTEN score for severity of illness 29
Age 40 years or older
Heart rate 120 beats per minute or higher
Malignancy present
Epidermal detachment affecting 10% or more of BSA
BUN level more than 28 mg/dL (10 mmol/L)
Serum bicarbonate level less than 20 mEq/L (20 mmol/L)
Serum glucose level more than 252 mg/dL (14 mmol/L)
Score 1 point for each item; mortality rate increases as score increases; 26 a score of 5 or more
may indicate 90% predicted mortality 18
Mortality is predominantly caused by multiorgan failure due to sepsis 38
Mortality rate for adults at 1 year 39
For Stevens-Johnson syndrome: 24%
For toxic epidermal necrolysis: 49%
For Stevens-Johnson syndrome/toxic epidermal necrolysis overlap: 43%
Mortality rates for children range from 0% to 16%, depending on severity 40
Among survivors, long-term complications are common 26
Screening and Prevention
Prevention
HLA-B genotype screening before prescribing anticonvulsants 14
HLA-B*15:02 screening before prescribing phenytoin or carbamazepine
HLA-B screening before prescribing allopurinol for gout 15 16
HLA-B*58:01 is linked to severe cutaneous adverse reactions
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 16 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Patients should be made aware of the risk of a recurrence with reexposure to the inciting
agent; reexposure should be avoided
REFERENCES
1: Creamer D et al: UK guidelines for the management of Stevens-Johnson syndrome/toxic
epidermal necrolysis in adults 2016. J Plast Reconstr Aesthet Surg. 69(6):e119-153, 2016
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/27287213)
2: Ellender RP et al: Clinical considerations for epidermal necrolysis. Ochsner J. 14(3):413-7,
2014
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/25249808)
3: Mukasa Y et al: Management of toxic epidermal necrolysis and related syndromes. Postgrad
Med J. 84(988):60-5, 2008
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/18322124)
4: McPherson T et al: British Association of Dermatologists' guidelines for the management of
Stevens-Johnson syndrome/toxic epidermal necrolysis in children and young people, 2018. Br J
Dermatol. 181(1):37-54, 2019
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/30829411)
5: Wetter DA et al: Clinical, etiologic, and histopathologic features of Stevens-Johnson
syndrome during an 8-year period at Mayo Clinic. Mayo Clin Proc. 85(2):131-8, 2010
View In Article | Cross Reference (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2813820/)
6: Harr T et al: Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare
Dis. 5:39, 2010
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/21162721)
7: National Organization for Rare Disorders: Stevens-Johnson Syndrome and Toxic Epidermal
Necrolysis. NORD website. Updated 2018. Accessed September 18, 2020.
https://rarediseases.org/rare-diseases/stevens-johnson-syndrome-and-toxic-epidermal-
necrolysis/
View In Article | Cross Reference (https://rarediseases.org/rare-diseases/stevens-johnson-
syndrome-and-toxic-epidermal-necrolysis/)
8: Lerch M et al: Current perspectives on Stevens-Johnson syndrome and toxic epidermal
necrolysis. Clin Rev Allergy Immunol. 54(1):147-76, 2018
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/29188475)
9: Letko E et al: Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 17 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
literature. Ann Allergy Asthma Immunol. 94(4):419-36; quiz 436-8, 456, 2005
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/15875523)
10: Dodiuk-Gad RP et al: Stevens-Johnson syndrome and toxic epidermal necrolysis: an update.
Am J Clin Dermatol. 16(6):475-93, 2015
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/26481651)
11: Ward KE et al: Severe adverse skin reactions to nonsteroidal antiinflammatory drugs: a
review of the literature. Am J Health System Pharm. 67(3):206-13, 2010
View In Article | Cross Reference (https://doi.org/10.2146/ajhp080603)
12: Oussalah A et al: Genetic variants associated with T-cell mediated cutaneous adverse drug
reactions: a Prisma-compliant systematic review--an EAACI position paper. Allergy. ePub, 2020
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/31899808)
13: Zeng T et al: Association of HLA-B*1502 allele with lamotrigine-induced Stevens-Johnson
syndrome and toxic epidermal necrolysis in Han Chinese subjects: a meta-analysis. Int J
Dermatol. 54(4):488-93, 2015
View In Article | Cross Reference (https://doi.org/10.1111/ijd.12570)
14: Karnes JH et al: Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline
for CYP2C9 and HLA-B genotypes and phenytoin dosing: 2020 update. Clin Pharmacol Ther.
ePub, 2020
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/32779747)
15: Saito Y et al: Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for
human leukocyte antigen B (HLA-B) genotype and allopurinol dosing: 2015 update. Clin
Pharmacol Ther. 99(1):36-7, 2016
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/26094938)
16: Hershfield MS et al: Clinical Pharmacogenetics Implementation Consortium guidelines for
human leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol Ther. 93(2):153-8,
2013
View In Article | Cross Reference (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3564416/)
17: Downey A et al: Toxic epidermal necrolysis: review of pathogenesis and management. J Am
Acad Dermatol. 66(6):995-1003, 2012
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/22169256)
18: Bastuji-Garin S et al: SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J
Invest Dermatol. 115(2):149-53, 2000
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/10951229)
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 18 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
19: Endorf FW et al: Toxic epidermal necrolysis clinical guidelines. J Burn Care Res. 29(5):706-
12, 2008
View In Article | Cross Reference (https://doi.org/10.1097/BCR.0b013e3181848bb1)
20: Bachot N et al: Differential diagnosis of severe cutaneous drug eruptions. Am J Clin
Dermatol. 4(8):561-72, 2003
View In Article | Cross Reference (https://www.ncbi.nlm.nih.gov/pubmed/12862499)
21: Casagranda A et al: Overlapping DRESS and Stevens-Johnson syndrome: case report and
review of the literature. Case Rep Dermatol. 9(2):1-7, 2017
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/28611628)
22: Hooten J et al: Updates on the management of autoimmune blistering diseases. Skin
Therapy Lett. 19(5):1-6, 2014
View In Article | Cross Reference (https://www.skintherapyletter.com/2014/19.5/1.html)
23: Ishii K: Importance of serological tests in diagnosis of autoimmune blistering diseases. J
Dermatol. 42(1):3-10, 2015
View In Article | Cross Reference (https://doi.org/10.1111/1346-8138.12703)
24: Fromowitz JS et al: Practical guidelines for the management of toxic epidermal necrolysis
and Stevens-Johnson syndrome. Int J Dermatol. 46(10):1092-4, 2007
View In Article | Cross Reference (https://doi.org/10.1111/j.1365-4632.2007.03277.x)
25: Maverakis E et al: Stevens-Johnson syndrome and toxic epidermal necrolysis standard
reporting and evaluation guidelines: results of a National Institutes of Health working group.
JAMA Dermatol. 153(6):587-92, 2017
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/28296986)
26: Nguyen DV et al: Human leukocyte antigen-associated severe cutaneous adverse drug
reactions: from bedside to bench and beyond. Asia Pac Allergy. 9(3):e20, 2019
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/31384575)
27: Barron SJ et al: Intravenous immunoglobulin in the treatment of Stevens-Johnson
syndrome and toxic epidermal necrolysis: a meta-analysis with meta-regression of observational
studies. Int J Dermatol. 54(1):108-15, 2015
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/24697283)
28: Zimmermann S et al: Systemic immunomodulating therapies for Stevens-Johnson
syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA
Dermatol. 153(6):514-22, 2017
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/28329382)
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 19 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
29: Roujeau J-C et al: Systematic review of treatments for Stevens-Johnson syndrome and toxic
epidermal necrolysis using the SCORTEN score as a tool for evaluating mortality. Ther Adv Drug
Saf. 2(3):87-94, 2011
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/25083204)
30: Ye LP et al: The effect of intravenous immunoglobulin combined with corticosteroid on the
progression of Stevens-Johnson syndrome and toxic epidermal necrolysis: a meta-analysis.
PLoS One. 11(11):e0167120, 2016
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/27902746)
31: Roujeau J-C et al: New evidence supporting cyclosporine efficacy in epidermal necrolysis. J
Invest Dermatol. 137(10):2047-9, 2017
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/28941473)
32: Schneider JA et al: Stevens-Johnson Syndrome and toxic epidermal necrolysis: a concise
review with a comprehensive summary of therapeutic interventions emphasizing supportive
measures. Adv Ther. 34(6):1235-44, 2017
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/28439852)
33: González-Herrada C et al: Cyclosporine use in epidermal necrolysis is associated with an
important mortality reduction: evidence from three different approaches. J Invest Dermatol.
137(10):2092-100, 2017
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/28634032)
34: Papp A et al: Treatment of toxic epidermal necrolysis by a multidisciplinary team: a review
of literature and treatment results. Burns. 44(4):807-15, 2018
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/29627131)
35: Finkelstein Y et al: Recurrence and outcomes of Stevens-Johnson syndrome and toxic
epidermal necrolysis in children. Pediatrics. 128(4):723-8, 2011
View In Article | Cross Reference (https://pediatrics.aappublications.org/content/128/4/723.long)
36: Kim H-I et al: Causes and treatment outcomes of Stevens-Johnson syndrome and toxic
epidermal necrolysis in 82 adult patients. Korean J Intern Med. 27(2):203-10, 2012
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/22707893)
37: Cabañas Weisz LM et al: Toxic epidermal necrolysis (TEN): acute complications and long-
term sequelae management in a multidisciplinary follow-up. J Plast Reconstr Aesthet Surg.
ePub, 2019
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/31481319)
38: Torres-Navarro I et al: Accuracy of SCORTEN to predict the prognosis of Stevens-Johnson
syndrome/toxic epidermal necrolysis: a systematic review and meta-analysis. J Eur Acad
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 20 dari 21
Stevens-Johnson syndrome and toxic epidermal necrolysis- ClinicalKey 07/03/21 19.15
Dermatol Venereol. ePub, 2019
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/31912590)
39: Dodiuk-Gad RP et al: Epidemiology of severe drug hypersensitivity. Semin Cutan Med Surg.
33(1):2-9, 2014
View In Article | Cross Reference (https://www.ncbi.nlm.nih.gov/pubmed/25037253)
40: Hsu DY et al: Pediatric Stevens-Johnson syndrome and toxic epidermal necrolysis in the
United States. J Am Acad Dermatol. 76(5):811-17.e4, 2017
View In Article | Cross Reference (https://pubmed.ncbi.nlm.nih.gov/28285784)
Copyright © 2021 Elsevier, Inc. All rights reserved.
https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-ab818f42-af79-4d28-9d11-a27f3ab0821b Halaman 21 dari 21
You might also like
- Stevens-Johnson Syndrome: Review of The Literature: Stitt CarolinaDocument4 pagesStevens-Johnson Syndrome: Review of The Literature: Stitt CarolinaNurul AfiahNo ratings yet
- Stevens-Johnson Syndrome and Abuse of Anabolic SteroidsDocument4 pagesStevens-Johnson Syndrome and Abuse of Anabolic SteroidsRafael SebastianNo ratings yet
- Steven Johnson SyndromeDocument13 pagesSteven Johnson SyndromeKhairul AnwarNo ratings yet
- Dermato - Emergemencies (Acute Blistering and Exfoliative Skin) Nyoman Suryawati ObjectiveDocument9 pagesDermato - Emergemencies (Acute Blistering and Exfoliative Skin) Nyoman Suryawati ObjectiveLong LieNo ratings yet
- Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A ReviewDocument6 pagesStevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A ReviewDede Rahman AgustianNo ratings yet
- Stevens-Johnson SyndromeDocument4 pagesStevens-Johnson SyndromeBelleNo ratings yet
- ClinRevSJsyndrome PDFDocument5 pagesClinRevSJsyndrome PDFMuhammad Nur Ardhi LahabuNo ratings yet
- Stevens-Johnson Syndrome (SJS)Document27 pagesStevens-Johnson Syndrome (SJS)Fitri wijayaNo ratings yet
- Print Jurnal 3Document7 pagesPrint Jurnal 3Amalia GrahaniNo ratings yet
- Stevens Johnson SyndromeDocument14 pagesStevens Johnson SyndromeGaemkyulyea KVn100% (1)
- Drug Induced-Stevens Johnson Syndrome: A Case ReportDocument26 pagesDrug Induced-Stevens Johnson Syndrome: A Case ReportdoktersandraNo ratings yet
- Stevens - Johnson SyndromeDocument16 pagesStevens - Johnson SyndromeElvis obajeNo ratings yet
- Manipal College of Medical Sciences Pokhara, Nepal: Erythema MultiformeDocument56 pagesManipal College of Medical Sciences Pokhara, Nepal: Erythema MultiformeVaibhav KrishnaNo ratings yet
- SJS (Jurnal)Document6 pagesSJS (Jurnal)FerryRoferdiNo ratings yet
- Toxic Epidermal Necrolysis: AetiologyDocument5 pagesToxic Epidermal Necrolysis: AetiologyreyhanrrNo ratings yet
- Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A ReviewDocument6 pagesStevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Reviewイアン リムホト ザナガNo ratings yet
- Stevens-Johnson Syndrome: Pathogenesis, Diagnosis, and ManagementDocument11 pagesStevens-Johnson Syndrome: Pathogenesis, Diagnosis, and ManagementsyalalaaalalaaaNo ratings yet
- SJS FixDocument11 pagesSJS FixVivi YolandhaNo ratings yet
- Sjogren'S Syndrome: A Review of Clinical Features, Diagnosis and Treatments AvailableDocument7 pagesSjogren'S Syndrome: A Review of Clinical Features, Diagnosis and Treatments AvailableGokull ShautriNo ratings yet
- TEN and SJSDocument8 pagesTEN and SJSreyhanrrNo ratings yet
- Stevens Johnson SyndromeDocument6 pagesStevens Johnson SyndromeAudrey LeonarNo ratings yet
- Vesicubullous Diseases & Mucocutaneous DisordersDocument23 pagesVesicubullous Diseases & Mucocutaneous Disordersshahlaraib001No ratings yet
- Laporan Kasus Tutorial 7Document36 pagesLaporan Kasus Tutorial 7ShaniaNo ratings yet
- 0130HMR - HMR 36 174Document8 pages0130HMR - HMR 36 174Devi_dvdvNo ratings yet
- Journal Reading PersentationDocument31 pagesJournal Reading PersentationKartikaEkaWulandariNo ratings yet
- E222 FullDocument3 pagesE222 FullromyNo ratings yet
- 11 217sindrom Stevens Johnson Diduga Akibat SiprofloksasinDocument5 pages11 217sindrom Stevens Johnson Diduga Akibat SiprofloksasinFebtyELyaNovikaNo ratings yet
- Nicotine-Induced Bullous Fixed Drug EruptionDocument3 pagesNicotine-Induced Bullous Fixed Drug Eruptionamal amanahNo ratings yet
- Background: Erythema MultiformeDocument11 pagesBackground: Erythema MultiformeseidooreikiNo ratings yet
- Toxic Epidermal Necrolysis and Stevens-Johnson Syndrome: Review ArticlesDocument5 pagesToxic Epidermal Necrolysis and Stevens-Johnson Syndrome: Review ArticlesFirdausi RiskiviawinandaNo ratings yet
- Putra,+18 Bmj.v5i1.274Document9 pagesPutra,+18 Bmj.v5i1.274noor hidayahNo ratings yet
- Background: Erythema MultiformeDocument23 pagesBackground: Erythema Multiformerizqi_cepiNo ratings yet
- Clinical Approach BlistersDocument25 pagesClinical Approach BlistersIsaacGonzalezNo ratings yet
- Erythema MultiformeDocument16 pagesErythema MultiformeGuen ColisNo ratings yet
- Kegawat Daruratan KulitDocument175 pagesKegawat Daruratan KulitDzulkifli SukriNo ratings yet
- BullousDocument3 pagesBullousLjubomirErdoglijaNo ratings yet
- Principles of Eye Management in Stevens-Johnson Syndrome: Mittanamalli S. SridharDocument5 pagesPrinciples of Eye Management in Stevens-Johnson Syndrome: Mittanamalli S. SridharFarah RNo ratings yet
- Case ReportDocument6 pagesCase ReportnafilahNo ratings yet
- Kuliah SMTR VDocument117 pagesKuliah SMTR VHorakhty PrideNo ratings yet
- Ten N Sjs ReviewDocument5 pagesTen N Sjs ReviewNantini GopalNo ratings yet
- Ijccm 25 575Document5 pagesIjccm 25 575Nandha KumarNo ratings yet
- Stevens-Johnson SyndromeDocument6 pagesStevens-Johnson SyndromeLau ColastreNo ratings yet
- Dress Syndrome With Mild Manifestations As A Diagnostic and Therapeutic Problem: Case ReportDocument6 pagesDress Syndrome With Mild Manifestations As A Diagnostic and Therapeutic Problem: Case ReportEllya Latifah IlyasNo ratings yet
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (Ten) Associated With Anti-Tubercular Drugs: A Case ReportDocument4 pagesStevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (Ten) Associated With Anti-Tubercular Drugs: A Case ReportIJAR JOURNALNo ratings yet
- Stevens-Johnson Syndrome CASEDocument38 pagesStevens-Johnson Syndrome CASEChristy Rose AgrisNo ratings yet
- Toxic Epidermal Necrolysis and Stevens Johnson Syndrome: Our Current UnderstandingDocument8 pagesToxic Epidermal Necrolysis and Stevens Johnson Syndrome: Our Current UnderstandingjoyfullNo ratings yet
- Cefixime Induced Stevens-Johnson Syndrome A Case Report and Review of LiteratureDocument2 pagesCefixime Induced Stevens-Johnson Syndrome A Case Report and Review of LiteratureDorainne JohnsonNo ratings yet
- Stevens-Johnson Syndrome (SJS)Document27 pagesStevens-Johnson Syndrome (SJS)Risang de ApuestoNo ratings yet
- Steven JohnsonDocument36 pagesSteven JohnsonPrincess Louise ViceralNo ratings yet
- Psoriasis: Posted: 02 Aug 2010 11:18 PM PDTDocument5 pagesPsoriasis: Posted: 02 Aug 2010 11:18 PM PDTScamb TrekNo ratings yet
- Behcet Disease AND Toxoplasmosis: by DR Shahzada Khan Tmo Eye A Ward HMCDocument67 pagesBehcet Disease AND Toxoplasmosis: by DR Shahzada Khan Tmo Eye A Ward HMCShahzada KhanNo ratings yet
- Em IvDocument4 pagesEm IvathiyafrNo ratings yet
- 1476 1503 1 PB PDFDocument8 pages1476 1503 1 PB PDFArief GusmanNo ratings yet
- Case Report: Subcorneal Pustular Dermatosis in Childhood: A Case Report and Review of The LiteratureDocument6 pagesCase Report: Subcorneal Pustular Dermatosis in Childhood: A Case Report and Review of The LiteratureIrsalinaHusnaAzwirNo ratings yet
- SSJ TenDocument5 pagesSSJ TenSelviana SudarmanNo ratings yet
- Drug Eruption: Bag/ SMF Ilmu Kesehatan Kulit Dan Kelamin, FK UNUD/ RSUP SanglahDocument40 pagesDrug Eruption: Bag/ SMF Ilmu Kesehatan Kulit Dan Kelamin, FK UNUD/ RSUP SanglahkadekapiklestariNo ratings yet
- Stevens-Johnson Syndrome Induced by Carbamazepine: PharmacologyDocument4 pagesStevens-Johnson Syndrome Induced by Carbamazepine: PharmacologyginNo ratings yet
- Toxic Epidermal Necrolysis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandToxic Epidermal Necrolysis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Cervical Disc Disease and Spondylosis: Rabia Qureshi, Jason A. Horowitz, Hamid HassanzadehDocument7 pagesCervical Disc Disease and Spondylosis: Rabia Qureshi, Jason A. Horowitz, Hamid HassanzadehMasithaNo ratings yet
- 03 Poli Crestani-2Document6 pages03 Poli Crestani-2MasithaNo ratings yet
- Annals of Medicine and SurgeryDocument5 pagesAnnals of Medicine and SurgeryMasithaNo ratings yet
- Lumbosacral Transitional Segments: An Interventional Spine Specialist's Practical ApproachDocument14 pagesLumbosacral Transitional Segments: An Interventional Spine Specialist's Practical ApproachMasithaNo ratings yet
- A S C C F e V H W H C: A C RDocument5 pagesA S C C F e V H W H C: A C RMasithaNo ratings yet
- Alam 2018Document11 pagesAlam 2018MasithaNo ratings yet
- NII-Electronic Library ServiceDocument3 pagesNII-Electronic Library ServiceMasithaNo ratings yet
- European Journal of Radiology: R. Siemund, M. Thurnher, P.C. SundgrenDocument8 pagesEuropean Journal of Radiology: R. Siemund, M. Thurnher, P.C. SundgrenMasithaNo ratings yet
- Computerized Medical Imaging and Graphics: Jianhua Yao, Joseph E. Burns, Hector Mu Noz, Ronald M. SummersDocument11 pagesComputerized Medical Imaging and Graphics: Jianhua Yao, Joseph E. Burns, Hector Mu Noz, Ronald M. SummersMasithaNo ratings yet
- Cholecystitis: Kaitlyn J. Kelly and Sharon Marie WeberDocument10 pagesCholecystitis: Kaitlyn J. Kelly and Sharon Marie WeberMasithaNo ratings yet
- Seminar: Disease Burden and EpidemiologyDocument12 pagesSeminar: Disease Burden and EpidemiologyMasithaNo ratings yet
- References: Alopecia Areata After Biologic Therapy: Report of A Case Related To AdalimumabDocument2 pagesReferences: Alopecia Areata After Biologic Therapy: Report of A Case Related To AdalimumabMasithaNo ratings yet
- Diagnosis of Double-Chambered Left Ventricle by Contrast Echocardiography: A Case ReportDocument6 pagesDiagnosis of Double-Chambered Left Ventricle by Contrast Echocardiography: A Case ReportMasithaNo ratings yet
- Diagnosisandmanagementof Psoriasisinchildren: Megha M. TollefsonDocument17 pagesDiagnosisandmanagementof Psoriasisinchildren: Megha M. TollefsonMasithaNo ratings yet
- COVID-19 With Acute Cholecystitis: A Case: Casereport Open AccessDocument4 pagesCOVID-19 With Acute Cholecystitis: A Case: Casereport Open AccessMasithaNo ratings yet
- European Journal of Oncology Nursing: A B A C A C D eDocument6 pagesEuropean Journal of Oncology Nursing: A B A C A C D eMasithaNo ratings yet
- Persistent Depressive Disorder (Dysthymia) - ClinicalKeyDocument13 pagesPersistent Depressive Disorder (Dysthymia) - ClinicalKeyMasithaNo ratings yet
- Review: Elisabeth Schramm, Daniel N Klein, Moritz Elsaesser, Toshi A Furukawa, Katharina DomschkeDocument12 pagesReview: Elisabeth Schramm, Daniel N Klein, Moritz Elsaesser, Toshi A Furukawa, Katharina DomschkeMasithaNo ratings yet
- Dysthymia: More Than Minor DepressionDocument6 pagesDysthymia: More Than Minor DepressionMasithaNo ratings yet
- Ageing Research Reviews: Annabel P. Matison, Karen A. Mather, Victoria M. Flood, Simone ReppermundDocument18 pagesAgeing Research Reviews: Annabel P. Matison, Karen A. Mather, Victoria M. Flood, Simone ReppermundMasithaNo ratings yet
- Clinical Neurology and NeurosurgeryDocument8 pagesClinical Neurology and NeurosurgeryMasithaNo ratings yet
- Pediatric Undernutrition: Table 30.1Document4 pagesPediatric Undernutrition: Table 30.1MasithaNo ratings yet
- Faktor Risiko Tuberkulosis Pada Anak: Muhammad S. D. Wijaya, Max F. J. Mantik, Novie H. RampenganDocument10 pagesFaktor Risiko Tuberkulosis Pada Anak: Muhammad S. D. Wijaya, Max F. J. Mantik, Novie H. RampenganMasithaNo ratings yet
- Cholera and Other: Vibrio InfectionsDocument8 pagesCholera and Other: Vibrio InfectionsMasithaNo ratings yet
- Jurnal Ilmiah Maksitek ISSN. 2655-4399 Vol. 5 No. 4 Desember 2020Document6 pagesJurnal Ilmiah Maksitek ISSN. 2655-4399 Vol. 5 No. 4 Desember 2020MasithaNo ratings yet
- Mapping The Burden of Cholera in Sub-Saharan Africa and Implications For Control: An Analysis of Data Across Geographical ScalesDocument8 pagesMapping The Burden of Cholera in Sub-Saharan Africa and Implications For Control: An Analysis of Data Across Geographical ScalesMasithaNo ratings yet
- JAK Inhibitors For Alopecia Areata: A Systematic Review and Meta-AnalysisDocument7 pagesJAK Inhibitors For Alopecia Areata: A Systematic Review and Meta-AnalysisMasithaNo ratings yet
- Cutaneous Bacterial Infections 3549Document17 pagesCutaneous Bacterial Infections 3549MasithaNo ratings yet
- Treatment of Severe Alopecia Areata With Baricitinib: Ase ReportDocument3 pagesTreatment of Severe Alopecia Areata With Baricitinib: Ase ReportMasithaNo ratings yet
- SKIN Tumors With Apocrine DifferentiationDocument101 pagesSKIN Tumors With Apocrine DifferentiationchinnnababuNo ratings yet
- Basic Principles of Real-Time PCRDocument19 pagesBasic Principles of Real-Time PCRMolecular_Diagnostics_KKUHNo ratings yet
- Z+blood TransfusionDocument16 pagesZ+blood TransfusionilhamaminsyaputraNo ratings yet
- Assessment of The Elderly Patient: Noel H. Ponce MDDocument33 pagesAssessment of The Elderly Patient: Noel H. Ponce MDlovelots1234No ratings yet
- Neuro Notes For The MCATDocument23 pagesNeuro Notes For The MCATadfhNo ratings yet
- DONA, A. ARVANITOYANNIS, I. S. - Health Risks of Genetically Modified Foods PDFDocument12 pagesDONA, A. ARVANITOYANNIS, I. S. - Health Risks of Genetically Modified Foods PDFLuciene AlvesNo ratings yet
- Basic Risk MeasurementDocument18 pagesBasic Risk Measurementgoku krishnaNo ratings yet
- Breast CancerDocument27 pagesBreast Cancerrikshya k c100% (3)
- Ethical Issues IVFDocument1 pageEthical Issues IVFBasinga OkinamNo ratings yet
- Planthormones 141025004114 Conversion Gate02 PDFDocument75 pagesPlanthormones 141025004114 Conversion Gate02 PDFSaiTimmarao100% (1)
- Anatomy & Physiology Eye: By: DR Abdul Salim Ismail Ophthalmology Department Hospital Pulau Pinang 8 July 2015Document35 pagesAnatomy & Physiology Eye: By: DR Abdul Salim Ismail Ophthalmology Department Hospital Pulau Pinang 8 July 2015muhammadridhwanNo ratings yet
- A Pattern Recognition Approach To Myopathy.15Document24 pagesA Pattern Recognition Approach To Myopathy.15Maryam NabNo ratings yet
- Dwnload Full Microbiology 1st Edition Wessner Solutions Manual PDFDocument35 pagesDwnload Full Microbiology 1st Edition Wessner Solutions Manual PDFyoreoakling.9vtk0y100% (8)
- Volume: 04 Issue: 06 - Nov-Dec 2023Document8 pagesVolume: 04 Issue: 06 - Nov-Dec 2023Central Asian StudiesNo ratings yet
- Psychosocial Case Study FormatDocument3 pagesPsychosocial Case Study FormatLoh Wei Chieh0% (1)
- Pathphys 2Document3 pagesPathphys 2zaydeeeeNo ratings yet
- Intellectual ExceptionalitiesDocument3 pagesIntellectual Exceptionalitiesapi-288159071No ratings yet
- 08 - Microorganisms and Their Effects On Living ThingsDocument5 pages08 - Microorganisms and Their Effects On Living ThingsMie IsaNo ratings yet
- Thalassemias and HemoglobinopathiesDocument63 pagesThalassemias and HemoglobinopathiesMahmod_Al_Bust_4830No ratings yet
- BorreliaDocument19 pagesBorreliaZetti Lenon Vallejos GomezNo ratings yet
- Turn Up The Heat - Unlock The Fat-Burning Power of Your MetabolismDocument378 pagesTurn Up The Heat - Unlock The Fat-Burning Power of Your MetabolismElvis Florentino96% (23)
- Cystic Fibrosis: Influenzae and The Most Important Is Pseudomonas Aeruginosa. Cystic Fibrosis Also Leads ToDocument3 pagesCystic Fibrosis: Influenzae and The Most Important Is Pseudomonas Aeruginosa. Cystic Fibrosis Also Leads ToLaramie ImNo ratings yet
- Zirconia ImplantsDocument15 pagesZirconia ImplantsmusatiiNo ratings yet
- Chap. 6 - 8 MATERNALDocument15 pagesChap. 6 - 8 MATERNALKaryll RomeroNo ratings yet
- NocardiaDocument3 pagesNocardiasujithas0% (1)
- Hille B-Second Edition (For Printing)Document617 pagesHille B-Second Edition (For Printing)matuskacarlosNo ratings yet
- Evaluation of A Diagnostic Flow Chart For Detection and Confirmation of Extended Spectrum B-Lactamases (ESBL) in EnterobacteriaceaeDocument11 pagesEvaluation of A Diagnostic Flow Chart For Detection and Confirmation of Extended Spectrum B-Lactamases (ESBL) in EnterobacteriaceaeRaffaharianggaraNo ratings yet
- UNSW Med Foundations Brief NotesDocument43 pagesUNSW Med Foundations Brief NotesXasdmanNo ratings yet
- BOTANY, Paper - II Model Paper: Part - IIIDocument3 pagesBOTANY, Paper - II Model Paper: Part - IIINishith ReddyNo ratings yet
- Hardy Weinberg Population GeneticsDocument3 pagesHardy Weinberg Population GeneticsMariaNo ratings yet