Professional Documents
Culture Documents
Naming Binary Compounds
Uploaded by
Lea SibayanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Naming Binary Compounds
Uploaded by
Lea SibayanCopyright:
Available Formats
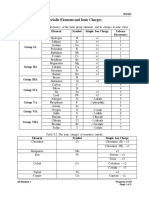
# of cation # of anion
cation anion needed to needed to
compound
symbol symbol make a make a formula name
neutral neutral
compound compound
magnesium ion
+ Mg2+ S2- 1 1 MgS Magnesium
sulfide
sulfide
Lithium ion +
iodide Li1+ I1- 1 1 LiI Lithium iodide
Potassium ion +
bromide K1+ Br1- 1 1 KBr Potassium
bromide
Calcium ion +
fluoride Ca2+ F1- 1 2 CaF2 Calcium fluoride
Beryllium ion +
oxide Be2+ O2- 1 1 BeO Beryllium oxide
Strontium ion +
sulfide Sr2+ S2- 1 1 SrS Strontium sulfide
Sodium ion +
bromide Na1+ Br1- 1 1 NaBr Sodium bromide
Aluminum ion +
chloride Al3+ Cl-1 1 3 AlCr3 Aluminum
chloride
Gallium ion +
iodide Ga3+ I1- 1 3 GaI3 Gallium (III)
iodide
Aluminum ion +
sulfide Al3+ S2- 2 3 Al2S3 Aluminum
sulfide
Gallium ion +
fluoride Ga3+ F1- 1 3 GaF3 Gallium (III)
fluoride
For each cation, list all the possible charges , and write the symbol and systematic
name for each charge
1. Iron Fe3+ iron(III) Fe2+ iron(II)
2. Copper Cu1+ Copper(I) Cu2+ Copper(II)
3. Cobalt Co2+ Cobalt(II) Co3+ Cobalt(III)
4. Tin Sb2+ Tin(II) Sb4+ Tin(IV)
5. Lead Pb2+ Lead (II) Pb4+ Lead(IV)
6. Mercury Hg1+ Mercury(I) Hg2+ Mercury (II)
Name the following binary compounds using Stock and Classical Method
CuCl2 Copper (II) chloride Cupric chloride
SbCl4 Tin (IV) chloride Stannic chloride
FeCl3 Iron (III) chloride Ferric chloride
CoF2 Cobalt (II) fluoride Cobaltous fluoride
CoF3 Cobalt (III) fluoride Cobaltic fluoride
PbO Lead (II) oxide Plumbous oxide
Co2S3 Cobalt (III) sulfide Cobaltic sulfide
Fe2S3 Iron (III) sulfide Ferric sulfide
You might also like
- Analysis of the New Metals: Titanium, Zirconium, Hafnium, Niobium, Tantalum, Tungsten and Their AlloysFrom EverandAnalysis of the New Metals: Titanium, Zirconium, Hafnium, Niobium, Tantalum, Tungsten and Their AlloysNo ratings yet
- Cations N AnionsDocument1 pageCations N AnionsgeelatifNo ratings yet
- Ionic Puzzle ActivityDocument4 pagesIonic Puzzle ActivityEngr Mumtaz0% (1)
- 10 - Ionic Bonding ActivityDocument4 pages10 - Ionic Bonding Activityapi-292000448No ratings yet
- Index Sa ChemistryDocument2 pagesIndex Sa ChemistryReiNo ratings yet
- Cation and Anion ListDocument1 pageCation and Anion ListAnizah AsiminNo ratings yet
- Valencies of Ions (For 9th and 10th)Document6 pagesValencies of Ions (For 9th and 10th)Irene AbhilashNo ratings yet
- Chemistry TablesDocument3 pagesChemistry Tableswvcs2gz9bbNo ratings yet
- Ions To KnowDocument2 pagesIons To KnowarouhsarahNo ratings yet
- Module 2 A Topic 1 Ion Formulae & Composite Formulae With DATADocument2 pagesModule 2 A Topic 1 Ion Formulae & Composite Formulae With DATASheikh Ahmad KamalNo ratings yet
- Common Ions and Ionic Charges 1+ 2+ 3+Document1 pageCommon Ions and Ionic Charges 1+ 2+ 3+xxpolxxNo ratings yet
- 5 - WS - Naming Ionic CompoundsDocument2 pages5 - WS - Naming Ionic Compoundsshabad700No ratings yet
- CationDocument2 pagesCationPuji RetnowatiNo ratings yet
- Cations: Ions and Charges Cations With Multiple ChargesDocument1 pageCations: Ions and Charges Cations With Multiple ChargesJohn Rey BayoguingNo ratings yet
- Common IonsDocument3 pagesCommon IonsabdallaaNo ratings yet
- Element Group Cation Element Group AnionsDocument3 pagesElement Group Cation Element Group AnionsCharlotte TanNo ratings yet
- Nomenclature of Inorganic Compounds: Report SheetDocument3 pagesNomenclature of Inorganic Compounds: Report SheetAEsmilingNo ratings yet
- Charge of ElementsDocument1 pageCharge of ElementsKagarine__LarousseNo ratings yet
- Types of CompoundsDocument15 pagesTypes of CompoundsJonard PedrosaNo ratings yet
- Notes IonsDocument1 pageNotes IonsVeda Faine TaburaNo ratings yet
- Valence SheetDocument1 pageValence SheetQueenie BelleNo ratings yet
- Metals With More Than One IonDocument2 pagesMetals With More Than One IonPATRICIA JULIANNE CASTAÑETO RIVERANo ratings yet
- Common IonsDocument2 pagesCommon Ionsnickloo55No ratings yet
- Anion Cation FormulaDocument1 pageAnion Cation FormulaharinistudentNo ratings yet
- Cations AnionsDocument2 pagesCations AnionsAngelica GementizaNo ratings yet
- Common Ions - Polyatomic IonsDocument2 pagesCommon Ions - Polyatomic IonsMak ItiNo ratings yet
- List of Common Cations: Name Symbol ChargeDocument4 pagesList of Common Cations: Name Symbol ChargernlpzcyNo ratings yet
- List of Common Cations Name Symbol ChargeDocument4 pagesList of Common Cations Name Symbol ChargernlpzcyNo ratings yet
- Writing Ionic FormulaeDocument6 pagesWriting Ionic FormulaeKhondokar TarakkyNo ratings yet
- CationsDocument2 pagesCationsOdd CatNo ratings yet
- F4 Che Basic (ANS)Document6 pagesF4 Che Basic (ANS)Chan ReneeNo ratings yet
- Valency TableDocument1 pageValency TableRitesh SinghNo ratings yet
- Fe CL Fe CL Fecl: Charge of The Ion Oxidation States of Transition MetalsDocument1 pageFe CL Fe CL Fecl: Charge of The Ion Oxidation States of Transition MetalsSuzaki KurushiNo ratings yet
- Chemical Formula NoteDocument1 pageChemical Formula NoteMuhammad Haikal Zainal100% (1)
- Names and Formulae of Common Ions IIDocument1 pageNames and Formulae of Common Ions IIlucyNo ratings yet
- Beginning Chemistry GuideDocument1 pageBeginning Chemistry GuideattyankeesNo ratings yet
- Names of RadicalsDocument4 pagesNames of RadicalsSnehin PoddarNo ratings yet
- Elements (Anions) Symbol Oxidation NO. Elements (Anions) Symbol Oxidation NODocument5 pagesElements (Anions) Symbol Oxidation NO. Elements (Anions) Symbol Oxidation NOJims Cudinyerah100% (1)
- p18 - p19 Compounds Ionic-AnswersDocument8 pagesp18 - p19 Compounds Ionic-Answersapi-423980580No ratings yet
- 5 Ion Chart 1Document1 page5 Ion Chart 1Mercury LineNo ratings yet
- Chemistry Ion Cheat SheetDocument2 pagesChemistry Ion Cheat SheetTiffany Gallina100% (4)
- DSE Chem Key TableDocument11 pagesDSE Chem Key TabletraceyNo ratings yet
- WS 1 Mole - FormulaDocument6 pagesWS 1 Mole - FormulaSEAW FUI MINGNo ratings yet
- Chemistry ReviewerDocument4 pagesChemistry ReviewerBhel San Pedro MarzanNo ratings yet
- Cations: Al Aluminium Fe Iron (III) CR Chromium (III)Document2 pagesCations: Al Aluminium Fe Iron (III) CR Chromium (III)NPNo ratings yet
- Chemical Bonding: Why Bond Anyway?Document45 pagesChemical Bonding: Why Bond Anyway?PutRi Charolin GintingNo ratings yet
- Formula Writing and Naming of CompoundsDocument1 pageFormula Writing and Naming of CompoundsMon ColinaNo ratings yet
- Katyon Ve Anyon TablosuDocument1 pageKatyon Ve Anyon TablosuhelenNo ratings yet
- FILE NO 3 Exercise 2 Chemical Formula Writing and Naming of Compounds RevDocument2 pagesFILE NO 3 Exercise 2 Chemical Formula Writing and Naming of Compounds RevEJ TaylanNo ratings yet
- Valency ChartDocument1 pageValency ChartAdam AzmiNo ratings yet
- Of Electrons That An Atom Either Gains or Loses in Order To Form A Chemical Bond With Another Atom. Example: MN 2+, O 2-, Fe 2+, CL - EtcDocument5 pagesOf Electrons That An Atom Either Gains or Loses in Order To Form A Chemical Bond With Another Atom. Example: MN 2+, O 2-, Fe 2+, CL - EtcAsif FarhanNo ratings yet
- Periodic Elements and Ionic ChargesDocument2 pagesPeriodic Elements and Ionic ChargeskjfhghjfggjfNo ratings yet
- Simple Chemistry Compound NamingDocument17 pagesSimple Chemistry Compound NamingBelinda AzaliaNo ratings yet
- Cations AnionsDocument1 pageCations AnionsTiviya Tarini ManiamNo ratings yet
- Cations and AnionsDocument2 pagesCations and AnionsG2 Atacador, Channa Keavy B.No ratings yet
- Formulae of Ions & Periodic TableDocument2 pagesFormulae of Ions & Periodic TableAn An LimNo ratings yet
- Chemical BondingDocument11 pagesChemical BondingXenia Mae FloresNo ratings yet
- Name of The Ion Ion Formulae ValenceDocument2 pagesName of The Ion Ion Formulae ValenceSara RashmiNo ratings yet
- 2051 MSDSDocument4 pages2051 MSDSNisa SutopoNo ratings yet
- Abraham, Yusuff - 2003 - Copper (II) Complexes of Embelin and 2-Aminobenzimidazole Encapsulated in Zeolite Y-Potential As Catalysts For RDocument9 pagesAbraham, Yusuff - 2003 - Copper (II) Complexes of Embelin and 2-Aminobenzimidazole Encapsulated in Zeolite Y-Potential As Catalysts For Rcukaasam123456No ratings yet
- SC Serie SC 4160Document8 pagesSC Serie SC 4160Anderson QuintãoNo ratings yet
- Liebermann Nitroso Test and Ninhydrin TestDocument4 pagesLiebermann Nitroso Test and Ninhydrin Testartemis MontecastroNo ratings yet
- Braja M Das - Geotechnical Engineering Handbook, Volumes 1 - 3-John Wiley & Sons (2002) - 3Document1 pageBraja M Das - Geotechnical Engineering Handbook, Volumes 1 - 3-John Wiley & Sons (2002) - 3Buliga MarianNo ratings yet
- Saturated Distilled Monoglycerides Variants in Gel-Form Cake EmulsifiersDocument9 pagesSaturated Distilled Monoglycerides Variants in Gel-Form Cake EmulsifiersKarla Susana Ramírez ArmendárizNo ratings yet
- Ecoprint On PaperDocument22 pagesEcoprint On PaperVeronica Rebirth100% (2)
- Cambridge O Level: Chemistry 5070/12Document16 pagesCambridge O Level: Chemistry 5070/12Raahin RahimNo ratings yet
- Physico-Chemical Parameters of Residual Water From Different Scouring Treatments of Hemp/Cotton FabricDocument7 pagesPhysico-Chemical Parameters of Residual Water From Different Scouring Treatments of Hemp/Cotton FabricHenry Pelayo RemacheNo ratings yet
- Worksheet: How Does A Catalyst Work?: Chemistry: CatalystsDocument2 pagesWorksheet: How Does A Catalyst Work?: Chemistry: Catalystskate remandabanNo ratings yet
- Sciencedirect: Journal of Photochemistry & Photobiology, B: Biology 202 (2020) 111676Document8 pagesSciencedirect: Journal of Photochemistry & Photobiology, B: Biology 202 (2020) 111676Anonymous DyytrQGxNo ratings yet
- MetalsDocument22 pagesMetalsNiamat UllahNo ratings yet
- Selleys Rp7-Aus GhsDocument9 pagesSelleys Rp7-Aus GhsdungdhtsNo ratings yet
- Evaluating An E-Nose Ability To Detect Biogas Plant Efficiency. A Case StudyDocument9 pagesEvaluating An E-Nose Ability To Detect Biogas Plant Efficiency. A Case StudyKentner Chavez CorreaNo ratings yet
- Covalent BondingDocument23 pagesCovalent BondingJames BorgNo ratings yet
- 20 MCQ - Chemical Bonding AS ChemistryDocument6 pages20 MCQ - Chemical Bonding AS ChemistryAijaz AhmedNo ratings yet
- Firestop Joint Spray CFS-SP WB: Technical Data ApplicationsDocument5 pagesFirestop Joint Spray CFS-SP WB: Technical Data ApplicationsBiprojit HoreNo ratings yet
- Bentona BP 183 B ChinaDocument2 pagesBentona BP 183 B Chinaoptimus_1404No ratings yet
- Manual & Automatic Chemical ControlDocument36 pagesManual & Automatic Chemical ControlKhaled SaadnehNo ratings yet
- Chemical Bonding and Molecular Structure Class 11 Notes Chemistry Chapter 11Document1 pageChemical Bonding and Molecular Structure Class 11 Notes Chemistry Chapter 11Jyoti JaiswalNo ratings yet
- Name Class Date: Twenty Electronic ConfigurationsDocument5 pagesName Class Date: Twenty Electronic ConfigurationsLeslie Vanessa CarrilloNo ratings yet
- Classton - CSV 1 Class Classton - KEY - CSV ZNDocument10 pagesClasston - CSV 1 Class Classton - KEY - CSV ZNJulyans Brousset CornejoNo ratings yet
- 10textural and Sensory M. MartinovicDocument12 pages10textural and Sensory M. MartinovicbabithyNo ratings yet
- Fermzilla-Leak-Instruction-Manual Final DraftDocument6 pagesFermzilla-Leak-Instruction-Manual Final DraftLagranderentreeeeNo ratings yet
- Roofing and Waterproofing: Standard Terminology Relating ToDocument9 pagesRoofing and Waterproofing: Standard Terminology Relating ToRONALD MUELLERNo ratings yet
- Electrolysis: Physical ChemistryDocument18 pagesElectrolysis: Physical ChemistryDavidson ChanNo ratings yet
- ASTM D8240-22e1Document4 pagesASTM D8240-22e1saderfende100% (1)
- Science Intervention Material (SIM) : in Grade 3Document11 pagesScience Intervention Material (SIM) : in Grade 3Vine BuenaventuraNo ratings yet
- Safe and Environmentally Friendly Large Power Transformers With EsterDocument4 pagesSafe and Environmentally Friendly Large Power Transformers With EsterbenlahnecheNo ratings yet
- Cohen R - Happer W 2015 - Fundamentals of Ocean PHDocument12 pagesCohen R - Happer W 2015 - Fundamentals of Ocean PHjms_martins6920No ratings yet