100% found this document useful (1 vote)
290 views13 pagesAcceptance Sampling Update - AQL LTPD
Acceptance Sampling Update
Uploaded by
Brandon YOUCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
100% found this document useful (1 vote)
290 views13 pagesAcceptance Sampling Update - AQL LTPD
Acceptance Sampling Update
Uploaded by
Brandon YOUCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
- Acceptance Sampling Update: Introduces the concept and importance of acceptance sampling in quality control and regulatory compliance.
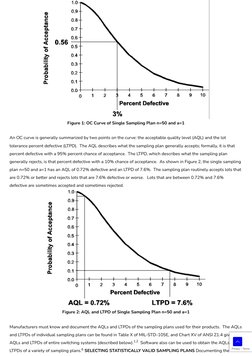
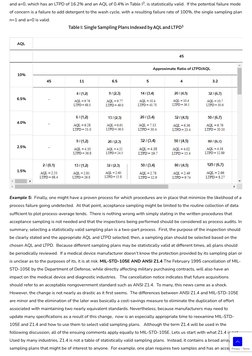
- Sampling Plan Details: Explains specific sampling plans and the construction of operating characteristic curves, providing examples with figures.
- Implementing Standards: Discusses standards and specifications for sampling plans to ensure consistency and compliance.
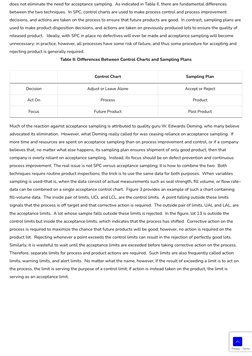
- Control and Sampling Plans: Compares control charts and sampling plans, highlighting their differences and application scenarios.
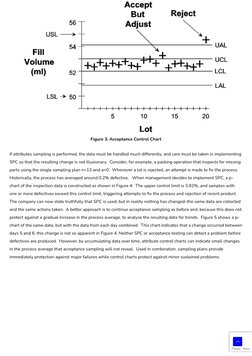
- Inspection Charts: Illustrates various charts used in inspection and control processes, including acceptance control and p-charts.
- Conclusion and References: Summarizes conclusions on acceptance sampling and provides references for further reading and research.