Professional Documents
Culture Documents
Physical Chemistry 2 - Midterm
Physical Chemistry 2 - Midterm
Uploaded by
Trung Võ0 ratings0% found this document useful (0 votes)
24 views5 pagesOriginal Title
Physical Chemistry 2_Midterm
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
24 views5 pagesPhysical Chemistry 2 - Midterm
Physical Chemistry 2 - Midterm
Uploaded by
Trung VõCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 5
International University - VNUHCM
School of Biotechnology/Department of Chemical Engineering
Midterm Examination
Date: November 03, 2021; Duration: 90 minutes
Open book; Online Laptops/Cell-phone are allowed.
SUBJECT
Name of course: Physical Chemistry 2 (ID: CHE1042IU)
Approval by —_the School of | Lecturer:
Biotechnology/Department of + Chemical | Signature
Engineering
Signature
Full name: Full name: Vu Bao Khanh
Proctor 1: Proctor 2.
Signature Signature
Full name: Vu Bao Khanh Full name:
STUDENT INFO
Student name:
Student ID:
INSTRUCTIONS: the total of point is 100 (equivalent to 30% of the course)
1. Purpose:
* Apply calculus in solving chemical kinetic problems.
+ Apply laws and theorems in processes to build an equation of rate reaction.
* Describe and explain fundamental concepts of physical chemistry, including those of
chemical kinetics, ideal and non-ideal solutions
+ Have a basic understanding of how physical models explain chemical properties and
reactivity.
2. Requirement:
* Please show your work to get the full credit.
HCMC National University Student Name:.
International University ‘Student ID:
* Write your answers directly on an A4 sheet. You can use more than one sheet if needed.
«Take photos of your answer sheet(s). Your ID (e.g, Student ID - Thé sinh vign, Personal ID
— Cn cuéc céng dan hay CMND) must be provided on the shaded area of the first page
using the template.
«Upload the photo(s) to Blackboard after completion.
+ Ifthere is any issue with the submission via Blackboard, please send your photos to
vbkhanh@hemiu.edu.vn
QUESTIONS
Q1. (20 marks)
The decomposition of ozone in the gas phase 20, —» 302 is catalyzed by chlorine. The
experimentally observed rate law for this reaction is:
1d{0,] _ 14/0
3
2
1
Dae 3 ae 7 KIC Flos]
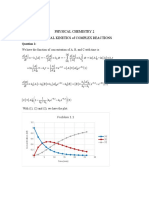
The reaction mechanism for this decomposition reaction has been proposed as follows:
(1)Ckh+0s “-» clo'+ coz (Initiation)
ke
(2) ClOs + Os —+ ClO" + O2
eo (Propagation)
(8) ClOs' + Os > ClOz' + 202
(4)2Clor “+ cCh+202
ke I (Termination)
(5)2ClO0 > Ch+O2
a. Identify intermediates.
b. Derive the rate law for ozone based on the above reaction mechanism by using the steady-
state approximation for intermediates. When does this rate law derived from the reaction
mechanism match with the rate law obtained from the experiment?
Q2, (20 marks)
For the reaction A --> products, the relationship between the concentration of A and reaction time was
collected and listed in the table below:
218
HCMC National University Student Name:.
International University ‘Student ID:
Timeh 0 1 2 3 4 5 6 7 8 9 10
[ALM 1.240.960 0.775 0.655 0.560 0.502 0.442 0.402 0.365 0.835 (0.310
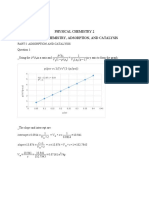
a. Test if this reaction follows zero-, first-, or second-order by making plots or using MS Excel to
perform linear regression. You have to test three cases even if you happen to guess the correct
the rate law on the first trial.
b. Determine the rate constant for the reaction
c. Calculate the half-life for the reaction based on the rate law you have determined.
d. At what time will the concentration of A be 0.380?
3. (20 marks)
The freezing point depression of stearic acid (8.0 g) with the presence of an unknown compound (1.0
9) can be expressed as follows:
6, = Kym
where @1 is the freezing point depression of stearic acid (4.5 °C), Kt is the freezing point depression
constant specific for a given solvent (4.50 °C-kg/mol for stearic acid), and m is the molality of the
solution. Calculate the molecular weight (g/mol) of the unknown compound?
Q4, (20 marks)
Using the Debye-Huckel limiting law, calculate the activity coefficients (y.) in 10° M solutions of HCl,
CaCle, and ZnSOs at 25 °C.
Q5. (20 marks)
The liquid-vapor equilibrium in the system, isopropyl aleohol-benzene, was studied over a range of
compositions at 25 °C. The vapor may be assumed to be an ideal gas. Let x: be the mole fraction of
the isopropyl alcohol in the liquid, and p1 be the partial pressure of the alcohol in the vapor. The data
are listed in the table below:
3/5
HCMC National University Student Name:.
Intemational University Student 0:
x 1.000 0.924 0.836
psmmHg 44.0 42.2 39.5
a. Calculate the rational activity of the isopropyl aleohol at x1 = 1.000, x1 = 0.924, and x: = 0.836.
b. Calculate the rational activity coefficient of the isopropyl alcohol at the three compositions in (a).
c. Atx1 = 0.836, calculate the amount by which the chemical potential of the alcohol differs from
that in an ideal solution.
- END -
415
HCMC National University
International University
‘Student 1D:
Name:
‘Signature:
5/5,
You might also like
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5813)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (844)
- PCP 2007/fa - Is: 1awlm CP 207L 1204 E (Ui3Document2 pagesPCP 2007/fa - Is: 1awlm CP 207L 1204 E (Ui3Trung VõNo ratings yet
- Had Cam Uac: Apsam VtyDocument2 pagesHad Cam Uac: Apsam VtyTrung VõNo ratings yet
- 6T Ceiua90Oy: VX 3.7864G Kao MlsDocument2 pages6T Ceiua90Oy: VX 3.7864G Kao MlsTrung VõNo ratings yet
- Physical Chemistry 2 Midterm Note I. Reaction Kinetic:: 1. Some ConceptsDocument9 pagesPhysical Chemistry 2 Midterm Note I. Reaction Kinetic:: 1. Some ConceptsTrung VõNo ratings yet
- PC 2 - Adsorb, Electro, CatalysDocument15 pagesPC 2 - Adsorb, Electro, CatalysTrung VõNo ratings yet
- Organic Chemistry 2Document5 pagesOrganic Chemistry 2Trung VõNo ratings yet
- A: Pkacte D Eo, 9: Licln JoockDocument2 pagesA: Pkacte D Eo, 9: Licln JoockTrung VõNo ratings yet
- Organic Chemistry 2: CL CL CL CL CL CL CL CL CLDocument3 pagesOrganic Chemistry 2: CL CL CL CL CL CL CL CL CLTrung VõNo ratings yet
- Take Home Practice - Sep 29 2021Document1 pageTake Home Practice - Sep 29 2021Trung VõNo ratings yet
- Chem Kinetic (Complex)Document5 pagesChem Kinetic (Complex)Trung VõNo ratings yet
- Organic Chemistry 2: Number of C ×Document5 pagesOrganic Chemistry 2: Number of C ×Trung VõNo ratings yet
- Chem Kinetic (Complex)Document6 pagesChem Kinetic (Complex)Trung VõNo ratings yet
- Chemical KineticsDocument9 pagesChemical KineticsTrung VõNo ratings yet
- Organic Chemistry 2 Homework 2: Vo Lam Hoai Trung BTCEIU19009Document4 pagesOrganic Chemistry 2 Homework 2: Vo Lam Hoai Trung BTCEIU19009Trung VõNo ratings yet
- Physical Chemistry 2 Chemical Kinetics: Mol - Min, K Mol - MinDocument16 pagesPhysical Chemistry 2 Chemical Kinetics: Mol - Min, K Mol - MinTrung VõNo ratings yet
- Rate Vs Concentartion (Reaction B) Rate Versus Concetration 2 (Reaction A)Document17 pagesRate Vs Concentartion (Reaction B) Rate Versus Concetration 2 (Reaction A)Trung VõNo ratings yet
- Chemical Kinetics of Complex Reactions - Sample Solution - Question 2Document3 pagesChemical Kinetics of Complex Reactions - Sample Solution - Question 2Trung VõNo ratings yet
- Chemical Kinetics of Complex ReactionsDocument3 pagesChemical Kinetics of Complex ReactionsTrung VõNo ratings yet
- Ideal and Non-Ideal SolutionsDocument2 pagesIdeal and Non-Ideal SolutionsTrung VõNo ratings yet
- Case StudiesDocument8 pagesCase StudiesTrung VõNo ratings yet