Professional Documents
Culture Documents
Chemical Kinetics of Complex Reactions
Uploaded by
Trung Võ0 ratings0% found this document useful (0 votes)
93 views3 pagesCopyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
93 views3 pagesChemical Kinetics of Complex Reactions
Uploaded by
Trung VõCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 3
CHEMICAL KINETICS of COMPLEX REACTIONS
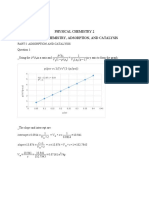
Question 1.
Series first order reaction:
Use Microsoft Excel to plot the concentrations of [A], [B], and [C] as a
function of reaction time for three cases:
1. [A]0 = 1.0 M, k1 = 0.2 min-1, k2 = 0.1 min-1
2. [A]0 = 1.0 M, k1 = 0.1 min-1, k2 = 0.2 min-1
3. [A]0 = 1.0 M, k1 = 0.1 min-1, k2 = 5.0 min-1
Which case can be assumed to be a steady-state-approximation?
Why?
Question 2.
Decomposition of di-2-methylpropan-2-yl peroxide produces
propanone and ethane:
and the generally accepted mechanism is:
1. Explain why this is not a chain reaction.
2. Using the steady-state-approximation to show that the reaction
follows the first order with respect to (CH3)3COOC(CH3)3, even
though it occurs in three consecutive steps. Formulate the rate of
reaction in terms of production of C2H6.
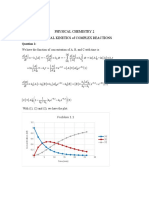
Question 3.
The reaction between nitric oxide (nitrogen monoxide) and oxygen
gives nitrogen dioxide according to the stoichiometric equation:
This reaction is experimentally found to be the second order in NO
and first order in O2. The following mechanisms can be proposed:
Mechanism A
Mechanism B
1. Assuming the steady state approximation, deduce the rate
expressions for each mechanism.
2. Under what conditions these mechanisms will fit the experimental
data and be kinetically equivalent? Hint: consider the denominator
and the mathematical and chemical significance of each term.
3. A further possibility is that the reaction occurs as an elementary
termolecular reaction: which mechanism(s) is the more likely?
Question 4.
This problem focuses on the difference between steady state
methods and the assumption of a pre-equilibrium for a reaction where
the first two steps are reversible.
The following mechanism is common to both inorganic and organic
reactions:
1. Carry out a steady state treatment, and find the conditions
under which this reaction would be:
(i) First order in both RX and Y¯ but inverse first order in X¯.
(ii) Simple first order in RX.
2. Now assume that the reversible reaction is at equilibrium, and
deduce the rate expression.
3. By comparing these two treatments in (1) and (2), find the
conditions under which the pre-equilibrium would be set up.
You might also like
- Solution: For A First-Order Reaction, The Following Rate Coefficients Were FoundDocument16 pagesSolution: For A First-Order Reaction, The Following Rate Coefficients Were FoundDeepak SharmaNo ratings yet
- Experiment 4: Effect of Concentration and Temperature On Rate of Reaction (Dissappearing Cross)Document24 pagesExperiment 4: Effect of Concentration and Temperature On Rate of Reaction (Dissappearing Cross)Malini RajeshNo ratings yet
- Introduction To Chemical Engineering CH 9Document14 pagesIntroduction To Chemical Engineering CH 9ABDO Moh.No ratings yet
- PhyChem II Simple Mixture PDFDocument39 pagesPhyChem II Simple Mixture PDFJupert Jasser AbellanaNo ratings yet
- Chem 112.1 - Exer 2 PostlabDocument7 pagesChem 112.1 - Exer 2 PostlabGerry Mark GubantesNo ratings yet
- Rr410802 Chemical Reaction Engineering IIDocument8 pagesRr410802 Chemical Reaction Engineering IISrinivasa Rao G100% (3)
- Catalyst Characterization TechniquesDocument8 pagesCatalyst Characterization TechniquesDaniel DadzieNo ratings yet
- Chemical Engineering Mass Balance CalculationsDocument16 pagesChemical Engineering Mass Balance CalculationsRose Dane Escobedo DiestaNo ratings yet
- Enzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisFrom EverandEnzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisRating: 4 out of 5 stars4/5 (2)
- Workshop IDocument6 pagesWorkshop IValentina GonzálezNo ratings yet
- Spectroscopy and ChromatographyDocument7 pagesSpectroscopy and ChromatographyPa GesNo ratings yet
- Chemical Reaction EngineeringDocument101 pagesChemical Reaction EngineeringGerard Toby CalixtoNo ratings yet
- Experimental Measurement of Boiling Point ElevationDocument33 pagesExperimental Measurement of Boiling Point Elevationsuleman205100% (3)
- Equilibrium Constant For Hydrolysis Lab6finalDocument9 pagesEquilibrium Constant For Hydrolysis Lab6finalapi-534386927No ratings yet
- Chapter 06 Phase Equilibria 4 PDF FreeDocument77 pagesChapter 06 Phase Equilibria 4 PDF FreeGabriel SilvaNo ratings yet
- Organic Chemistry Exercise PDFDocument34 pagesOrganic Chemistry Exercise PDFBrightMoonNo ratings yet
- Refresher 1-Answer KeyDocument4 pagesRefresher 1-Answer KeyDzyl Karee F. AllenNo ratings yet
- Tie Substance For Material BalanceDocument7 pagesTie Substance For Material BalanceMukesh Tiwari100% (3)
- Isothermal Reactor DesignDocument3 pagesIsothermal Reactor Designنزار الدهاميNo ratings yet
- Set3ans 10Document5 pagesSet3ans 10amalinaishahNo ratings yet
- NMR Kinetics: Study of A Reversible Hydrolysis ReactionDocument8 pagesNMR Kinetics: Study of A Reversible Hydrolysis ReactionOldbooklover100% (2)
- Thermo FinalDocument66 pagesThermo Finalsossydj75% (4)
- An Industrial Wastewater Contains 10 MG L ChlorophenolDocument5 pagesAn Industrial Wastewater Contains 10 MG L ChlorophenolsumitNo ratings yet
- Introduction To Chemical Engineering CH 7Document19 pagesIntroduction To Chemical Engineering CH 7정미학 / 학생 / 자유전공학부No ratings yet
- Solution For "Introduction To Chemical Engineering" Chapter 12Document6 pagesSolution For "Introduction To Chemical Engineering" Chapter 12jiholee1117No ratings yet
- Chap 12-13Document5 pagesChap 12-13noviNo ratings yet
- CH 6701 Cre IiDocument230 pagesCH 6701 Cre IiVaibhav Gupta100% (1)
- Chapter 9Document17 pagesChapter 9JajejijojuNo ratings yet
- rr310802 Chemical Engineering Thermodynamics IIDocument8 pagesrr310802 Chemical Engineering Thermodynamics IISRINIVASA RAO GANTANo ratings yet
- Complex Reactions: Dr. Rer. Nat. Deni RahmatDocument38 pagesComplex Reactions: Dr. Rer. Nat. Deni Rahmathelenismaya100% (1)
- Solution For "Introduction To Chemical Engineering" Chapter 11Document8 pagesSolution For "Introduction To Chemical Engineering" Chapter 11jiholee1117No ratings yet
- Ccb2053 Tutorial 1Document1 pageCcb2053 Tutorial 1eja70No ratings yet
- Catalyst Characterization - W6Document33 pagesCatalyst Characterization - W6Safitri WulansariNo ratings yet
- 12 Chemistry Notes Ch04 Chemical KineticsDocument4 pages12 Chemistry Notes Ch04 Chemical KineticssrideviNo ratings yet
- Tutorial Reactive SystemsDocument33 pagesTutorial Reactive Systemssiti azilaNo ratings yet
- (4.1) Laminar Premixed FlameDocument31 pages(4.1) Laminar Premixed Flameمصطفى العباديNo ratings yet
- (P01, C01, C02, C2, C3) : Confidential EH/JUN 2014/CHE584/594Document11 pages(P01, C01, C02, C2, C3) : Confidential EH/JUN 2014/CHE584/594Addison JuttieNo ratings yet
- Principles of Chemical EquilibriumDocument17 pagesPrinciples of Chemical EquilibriumkaditasookdeoNo ratings yet
- 10 PDFDocument23 pages10 PDFTysir SarhanNo ratings yet
- Tutorial - Transport Eqn, EosDocument15 pagesTutorial - Transport Eqn, Eossiti azilaNo ratings yet
- Chem Xi Chap 2, Worksheet 3Document4 pagesChem Xi Chap 2, Worksheet 3nazish kiranNo ratings yet
- Chapter 2 - Data InterpretationDocument24 pagesChapter 2 - Data InterpretationPHƯƠNG ĐẶNG YẾNNo ratings yet
- Tutorial 4 Achem PDFDocument12 pagesTutorial 4 Achem PDFyassinroslanNo ratings yet
- IB Chemistry Empirical Formula WorksheetDocument2 pagesIB Chemistry Empirical Formula WorksheetSherida GibbsNo ratings yet
- BT HPTDocument31 pagesBT HPTLinh NguyenNo ratings yet
- Lab Report 3Document9 pagesLab Report 3ainnorNo ratings yet
- Ench 529 Virtual Lab (E5) InstructionsDocument2 pagesEnch 529 Virtual Lab (E5) Instructionsench501No ratings yet
- Lecture 9 - Collection and Analysis of Rate DataDocument13 pagesLecture 9 - Collection and Analysis of Rate DataSabrina AzharNo ratings yet
- ChBE 3210A Transport Processes II Exam #2 Spring 2007Document6 pagesChBE 3210A Transport Processes II Exam #2 Spring 2007Abishek KasturiNo ratings yet
- Activity Coefficient ThermodynamicsDocument6 pagesActivity Coefficient ThermodynamicsPavan TejNo ratings yet
- CHE 304 Problem Set 8 SolutionsDocument5 pagesCHE 304 Problem Set 8 SolutionsAgustina Evania DewiNo ratings yet
- CH E 511A: Separation Processes and Introduction To Particulate Technology LeachingDocument8 pagesCH E 511A: Separation Processes and Introduction To Particulate Technology LeachingKhayie VictorianoNo ratings yet
- Effect of Solvent Polarity on SN1 Reaction RateDocument7 pagesEffect of Solvent Polarity on SN1 Reaction RateangelbenavidezNo ratings yet
- Collection and Analysis of Rate DataDocument24 pagesCollection and Analysis of Rate DataAfs IkhlasNo ratings yet
- Reaction KineticsDocument37 pagesReaction KineticsNurshuhada NordinNo ratings yet
- Potentiometric TitulationsDocument18 pagesPotentiometric Titulationslilipu0% (1)
- Physical 10Document2 pagesPhysical 10Rodriguez RommelNo ratings yet
- Punjab College Pattoki: Spring 2021: Course Outline Bs Program Semester 6ThDocument8 pagesPunjab College Pattoki: Spring 2021: Course Outline Bs Program Semester 6ThFareeha ShakeelNo ratings yet
- Counter-Current Extraction: An Introduction to the Design and Operation of Counter-Current ExtractorsFrom EverandCounter-Current Extraction: An Introduction to the Design and Operation of Counter-Current ExtractorsNo ratings yet
- VoLm toa rmn heat transfer analysisDocument2 pagesVoLm toa rmn heat transfer analysisTrung VõNo ratings yet
- PCP 2007/fa - Is: 1awlm CP 207L 1204 E (Ui3Document2 pagesPCP 2007/fa - Is: 1awlm CP 207L 1204 E (Ui3Trung VõNo ratings yet
- Had Cam Uac: Apsam VtyDocument2 pagesHad Cam Uac: Apsam VtyTrung VõNo ratings yet
- Heat Distribution in Infinite Slabs and CylindersDocument1 pageHeat Distribution in Infinite Slabs and CylindersTrung VõNo ratings yet
- PHYSICAL CHEMISTRY 2 MIDTERM NOTESDocument9 pagesPHYSICAL CHEMISTRY 2 MIDTERM NOTESTrung VõNo ratings yet
- Organic Chemistry 2Document5 pagesOrganic Chemistry 2Trung VõNo ratings yet
- Adsorption and Catalysis Reaction RatesDocument15 pagesAdsorption and Catalysis Reaction RatesTrung VõNo ratings yet
- A: Pkacte D Eo, 9: Licln JoockDocument2 pagesA: Pkacte D Eo, 9: Licln JoockTrung VõNo ratings yet
- Hw2 - Vo Lam Hoai Trung-Btceiu19009Document4 pagesHw2 - Vo Lam Hoai Trung-Btceiu19009Trung VõNo ratings yet
- Relationship between natural log of concentration and voltage in electrochemical cellDocument6 pagesRelationship between natural log of concentration and voltage in electrochemical cellTrung VõNo ratings yet
- Organic Chemistry 2: CL CL CL CL CL CL CL CL CLDocument3 pagesOrganic Chemistry 2: CL CL CL CL CL CL CL CL CLTrung VõNo ratings yet
- Chemical KineticsDocument9 pagesChemical KineticsTrung VõNo ratings yet
- Organic Chemistry 2: CL CL CL CL CL CL CL CL CLDocument3 pagesOrganic Chemistry 2: CL CL CL CL CL CL CL CL CLTrung VõNo ratings yet
- Take Home Practice-Vo Lam Hoai Trung-BTCEIU19009Document4 pagesTake Home Practice-Vo Lam Hoai Trung-BTCEIU19009Trung VõNo ratings yet
- Organic Chemistry 2: Number of C ×Document5 pagesOrganic Chemistry 2: Number of C ×Trung VõNo ratings yet
- Organic Chemistry 2 Homework 2: Vo Lam Hoai Trung BTCEIU19009Document4 pagesOrganic Chemistry 2 Homework 2: Vo Lam Hoai Trung BTCEIU19009Trung VõNo ratings yet
- Chem Kinetic (Complex)Document5 pagesChem Kinetic (Complex)Trung VõNo ratings yet
- Ideal and Non-Ideal SolutionsDocument2 pagesIdeal and Non-Ideal SolutionsTrung VõNo ratings yet
- Physical Chemistry 2 Chemical Kinetics: Mol - Min, K Mol - MinDocument16 pagesPhysical Chemistry 2 Chemical Kinetics: Mol - Min, K Mol - MinTrung VõNo ratings yet
- First-Order Reaction Kinetics Concentration Over TimeDocument6 pagesFirst-Order Reaction Kinetics Concentration Over TimeTrung VõNo ratings yet
- Case Study Self-SolvingDocument3 pagesCase Study Self-SolvingTrung VõNo ratings yet
- Chemical Kinetics of Complex Reactions - Sample Solution - Question 2Document3 pagesChemical Kinetics of Complex Reactions - Sample Solution - Question 2Trung VõNo ratings yet
- Rate Vs Concentartion (Reaction B) Rate Versus Concetration 2 (Reaction A)Document17 pagesRate Vs Concentartion (Reaction B) Rate Versus Concetration 2 (Reaction A)Trung VõNo ratings yet
- Case StudiesDocument8 pagesCase StudiesTrung VõNo ratings yet