Professional Documents
Culture Documents
Infinosis FSH IN027704 en
Uploaded by
Meditech visionbdOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Infinosis FSH IN027704 en
Uploaded by
Meditech visionbdCopyright:
Available Formats
IN0277042105V1
POC FSH infinosis™
Follicle-stimulating Hormone
REF: IN027704 25 tests • The test cassette should remain in its original sealed pouch until ready to use. Mix the specimen well with buffer for 5-10 seconds by tapping or inverting the
Do not use it if the pouch is damaged or the seal is broken. tube.
Intended use • Do not use reagents beyond the labeled expiry date. Step 4: Loading
The Infinosis™ FSH is an immunofluorescence-based lateral flow assay for the in • Do not mix or use components from kits with different Lots. Add 80 μL of sample mixture and load it onto the sample well of the test cassette.
vitro quantitative determination of follicle-stimulating hormone (FSH) in human • Don’t use Test Cassette if its Lot does not match with ID Chip that is inserted
Step 5: Testing
serum or plasma. onto the instrument.
Put the Test Cassette on the operation platform. 15 minutes later, insert the Test
• The Infinosis™ FSH assay should be used with Infinosis™ 2020 FIA analyzer.
Cassette onto the Cassette Holder and click “Test”. The result will show in the
Summary1-5 • The tests should be applied by professionally trained staff working in certified
display and print out when click “Print”.
FSH (follicle stimulating hormone), together with LH (luteinizing hormone), laboratories at some remove from the patient and clinic at which the sample is
belongs to the gonadotropin family. FSH and LH regulate and stimulate the growth taken by qualified medical personnel. Please refer to the Infinosis™ 2020 FIA analyzer Operation Manual for details.
and function of the gonads (ovaries and testes) synergistically. Like LH, TSH and • Infinosis™ FSH assay is single use only. Do not re-use it.
hCG, FSH is a glycoprotein consisting of two subunits (α- and β‐chains). Its • The Test Cassette and Analyzer should be used away from vibration and Limitations - interference
molecular weight is approx. 32000 daltons. In women, the gonadotropins act magnetic field. During normal usage, the Test Cassette may introduce minute • The assay is unaffected by icterus (bilirubin < 1094 μmol/L or < 64 mg/dL),
within the hypothalamus‐pituitary‐ovary regulating circuit to control the menstrual vibration, which should be regarded as normal. hemolysis (Hb < 0.621 mmol/L or < 1.0 g/dL), lipemia (Intralipid < 1900 mg/dL)
cycle. • Use separate clean pipette tips and detector buffer vials for different specimens. and biotin (< 246 nmol/L or < 60 ng/mL).
FSH and LH are released in pulses from the gonadotropic cells of the anterior The pipette tips and detector buffer vials should be used for one specimen only. • Criterion: Recovery within ± 10 % of initial value.
pituitary. The levels of the circulating hormones are controlled by steroid • Do not smoke, eat, or drink in areas in which specimens or kit reagents are • Samples should not be taken from patients receiving therapy with high biotin
hormones via negative feedback to the hypothalamus. In the ovaries FSH, handled. doses (i.e. > 5 mg/day) until at least 8 hours
together with LH, stimulates the growth and maturation of the follicle and hence • Blood specimens, used test cassettes, pipette tips and detector buffer vials are • following the last biotin administration.
also the biosynthesis of estrogens in the follicles. The FSH level shows a peak at potentially infectious. Proper laboratory safety techniques, handing and • No interference was observed from rheumatoid factors up to a concentration of
mid-cycle, although this is less marked than with LH. Due to changes in ovarian disposal methods should be followed in accordance with standard procedures 2250 IU/mL.
function and reduced estrogen secretion, high FSH concentrations occur during and relevant regulations observed by microbiological hazard materials. • There is no high-dose hook effect at FSH concentrations up to 2000 mIU/mL.
menopause. In men, FSH serves to induce spermatogonium development. • The results should be interpreted by the physician along with clinical findings • In vitro tests were performed on 17 commonly used pharmaceuticals. No
Determination of the FSH concentration is used in the elucidation of dysfunctions and other laboratory test results. interference with the assay was found.
within the hypothalamus‐pituitary‐gonads system. The determination of FSH in • The test will be applied on a routine basis and not in emergency situations. In rare cases, interference due to extremely high titers of antibodies to analyte‐
conjunction with LH is utilized for the following indications: congenital diseases specific antibodies, streptavidin or ruthenium can occur. These effects are
with chromosome aberrations, polycystic ovaries (PCO), amenorrhea (causes), Storage minimized by suitable test design.
and menopausal syndrome. Depressed gonadotropin levels in men occur in • Store the Sample buffer at 4-30°C. The buffer is stable up to 24 months. • For diagnostic purposes, the results should always be assessed in conjunction
azoospermia. • Store Infinosis™ test cassette at 4-30°C, shelf life is up to 24 months. with the patient’s medical history, clinical examination and other findings.
• Test cassette should be used within 1 hour after opening the pack.
Test principle Limits and ranges
Sandwich principle. Total duration of assay: 15 minutes Specimen collection and preparation Measuring range
Sample is added to the sample well of the test, then the fluorescence-labeled • The test can be performed with either serum or plasma. 1.0‐150 mIU/mL (defined by the lower detection limit and the maximum of the
detector anti-FSH antibody binds to FSH antigen in blood specimen. As the • Collect serum samples in accordance with correct medical practices. master curve). Values below the lower detection limit are reported as < 1.0 mIU/
sample mixture migrates on the nitrocellulose matrix of test strip by capillary • Using standard phlebotomy procedure, collect a venipuncture whole blood mL. Values above the measuring range are reported as > 150 mIU/mL.
action, the complexes of detector antibody and LH are captured to anti-FSH specimen using a blood collection tube. If collecting plasma use a blood
antibody that has been immobilized on test strip. collection tube containing suitable anticoagulant (EDTA recommended). Lower limits of measurement
The more FSH antigen is in blood specimen, the more complexes are • Separate the serum/plasma from blood as soon as possible to avoid hemolysis. Lower detection limit
accumulated on test strip. Signal intensity of fluorescence of detector antibody • Test should be performed immediately after the specimens have been collected. Lower detection limit: 1.0 mIU/mL
reflects amount of FSH captured and Infinosis™ FIA analyzer shows FSH Do not leave the specimens at room temperature for prolonged periods. The detection limit represents the lowest analyte level that can be distinguished
concentrations in blood specimen. The default results unit of Infinosis™ FSH test Specimens may be stored at 2-8°C for up to 3 days. For long-term storage, from zero. It is calculated as the value lying two standard deviations above that of
is displayed as x mIU/mL from Infinosis™ FIA analyzer. specimens should be kept below -20°C. the lowest standard (master calibrator, standard 1 + 2 SD, repeatability study, n =
20).
Reagents Quality control
Materials provided Each Infinosis™ FSH test cassette contains internal control that satisfies routing Expected values
• Test cassette, 25 pcs, individually packaged quality control requirements. This internal control is performed each time a patient Men: 1.1 - 14.4 mIU/mL
• ID chip, 1 pcs sample is tested. This control indicates that the test cartridge was inserted and Women:
• Sample buffer, 25 vials read properly by Infinosis™ 2020 FIA analyzer. An invalid result from the internal • Follicular phase: 3.2 - 12.7 mIU/mL
• IFU, 1 copy control causes an error message on Infinosis™ 2020 FIA analyzer indicating that • Ovulation phase: 4.5 - 22.8 mIU/mL
the test should be repeated. • Luteal phase: 1.2 - 8.9 mIU/mL
Materials required (but not provided) • Postmenopause: 22 - 146 mIU/mL
• Infinosis™ 2020 FIA analyzer Test procedure
• Transfer pipette set (100 μL size) Refer to Infinosis™ 2020 FIA analyzer Operation Manual for the complete LH/FSH quotient: Quotients have been calculated from the results obtained with
• Alcohol pads instructions on use of the test. The test should be operated in room temperature. the Infinosis™ LH assay and the Infinosis™ FSH assay in the samples of healthy
• Centrifuge (for plasma and serum only) Step 1: Preparation women of child‐bearing age. The following medians have been calculated:
• Timer Check/insert ID Chip into the analyzer.
Follicular phase: 0.89 (n = 331)
Precautions and warnings Step 2: Sampling Luteal phase: 1.15 (n = 306)
Add 20 μL of plasma/serum to the buffer tube.
• For in vitro diagnostic use only.
• Carefully follow the instructions and procedures described in this instructions Step 3: Mixing Each laboratory should investigate the transferability of the expected values to its
before testing. own patient population and if necessary determine its own reference ranges.
202105 V1 English 1/2 Infinosis™ FSH/IFU
IN0277042105V1
POC FSH infinosis™
Follicle-stimulating Hormone
Specific performance data
Representative performance data are given below. Results obtained in individual
laboratories may differ.
Precision
diasino
Intra-assay DiaSino Laboratories Co., Ltd
Determined by using 10 tests in the same batch to test with FSH control, CV ≤ No.68, Jingnansi Road, National Eco & Tech Development Area
15% Zhengzhou, China. 450000
Inter-assay Technical Support: ts@diasino.com
Determined by using 3 tests in 3 random and continuous batches to test with FSH www.diasino.com
control, CV ≤ 20%
Method comparison
A comparison of the FSH assay (y) with the Roche Elecsys FSH (x) using clinical
samples gave the following correlations:
Number of samples measured: 138
Linear regression
y = 0.9874x + 0.083
r = 0.9855
Analytical specificity
For the monoclonal antibodies used, the following cross-reactivities were found:
LH 0.045%, FSH 0.01%; hGH and hCG no cross-reactivity.
References
1. Johnson MR, Carter G, Grint C, et al. Relationship between ovarian steroids,
gonadotropin and relaxin during the menstrual cycle. Acta Endocrinol
1983;129/2:121-125.
2. Beastall GH, Ferguson KM, O’Reilly DSJ, et al. Assays for follicle stimulating
hormone and luteinizing hormone: Guidelines for the provision of a clinical
biochemistry service. Ann Clin Biochem 1987;24:246-262.
3. Runnebaum B, Rabe T. Gynäkologische Endokrinologie und
Fortpflanzungsmedizin Springer Verlag 1994. Band 1:17,253-255, Band
2:152-154,360,348. ISBN 3-540-57345-3, ISBN 3-540-57347-X.
4. Schmidt-Mathiesen H. Gynäkologie und Geburtshilfe. Schattauer Verlag 1992.
5. Scott MG, Ladenson JH, Green ED, et al. Hormonal evaluation of female
infertility and reproductive disorders. Clin Chem 1989;35:620-630.
In vitro diagnostic Refer to instruction
use for use
Expiry date Manufacturing date
Batch number Test per kit
Catalog number Do not re-use
Store between
Manufacturer
4-30℃
202105 V1 English 2/2 Infinosis™ FSH/IFU
You might also like
- Infinosis HCG - IN027702 - enDocument2 pagesInfinosis HCG - IN027702 - enMeditech visionbdNo ratings yet
- Infinosis Total IgE IN067705 enDocument2 pagesInfinosis Total IgE IN067705 enMeditech visionbdNo ratings yet
- Infinosis Troponin I IN047701 enDocument2 pagesInfinosis Troponin I IN047701 enMeditech visionbdNo ratings yet
- Infinosis T3-In017702 enDocument2 pagesInfinosis T3-In017702 enMeditech visionbdNo ratings yet
- Infinosis NT-proBNP IN047705 enDocument2 pagesInfinosis NT-proBNP IN047705 enMeditech visionbdNo ratings yet
- Infinosis PCT IN057701 enDocument2 pagesInfinosis PCT IN057701 enMeditech visionbdNo ratings yet
- Infinosis LH IN027703 enDocument2 pagesInfinosis LH IN027703 enMeditech visionbdNo ratings yet
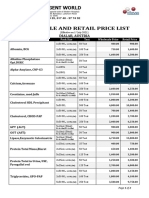
- Price List Wholesale and Retail (June-2021)Document2 pagesPrice List Wholesale and Retail (June-2021)Meditech visionbdNo ratings yet
- Welq T KV Ri Avb ': Qvî/Qvîxi BVG T Gvzvi BVG T Wczvi BVG T Ivj Bs TDocument19 pagesWelq T KV Ri Avb ': Qvî/Qvîxi BVG T Gvzvi BVG T Wczvi BVG T Ivj Bs TMeditech visionbdNo ratings yet
- Qvî/Qvîxi BVG T Ivj Bs Köbx WelqDocument13 pagesQvî/Qvîxi BVG T Ivj Bs Köbx WelqMeditech visionbdNo ratings yet
- Febrile AntigenbnkggDocument4 pagesFebrile AntigenbnkggMeditech visionbdNo ratings yet
- Infinosis HbA1c IN067701 enDocument2 pagesInfinosis HbA1c IN067701 enMeditech visionbdNo ratings yet
- Biochemistry Chema, Italy: (IFCC) (IFCC) (IFCC)Document2 pagesBiochemistry Chema, Italy: (IFCC) (IFCC) (IFCC)Meditech visionbdNo ratings yet
- SUBJECT:-APPOINTMENT TO THE POST OF Executive (Sales & Marketing)Document2 pagesSUBJECT:-APPOINTMENT TO THE POST OF Executive (Sales & Marketing)Meditech visionbdNo ratings yet
- M T Jute Trading: Count & Quality: 10/1 LBS 1 PLY HessianDocument4 pagesM T Jute Trading: Count & Quality: 10/1 LBS 1 PLY HessianMeditech visionbdNo ratings yet
- Price Quotation For Pathological Reagents. Sir,: (IFCC) (IFCC) (IFCC)Document1 pagePrice Quotation For Pathological Reagents. Sir,: (IFCC) (IFCC) (IFCC)Meditech visionbdNo ratings yet
- LP F060 CH 6 X 10 ML LP F125 CH 5 X 25 ML: in Vitro Diagnostic Medical DeviceDocument1 pageLP F060 CH 6 X 10 ML LP F125 CH 5 X 25 ML: in Vitro Diagnostic Medical DeviceMeditech visionbdNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Technical Guidance Notes M2 Monitoring of Stack Emissions To Air Environment Agency UKDocument89 pagesTechnical Guidance Notes M2 Monitoring of Stack Emissions To Air Environment Agency UKheljoalNo ratings yet
- Ft4 Ii: Free ThyroxineDocument4 pagesFt4 Ii: Free ThyroxinehairiNo ratings yet
- Beckman PDFDocument7 pagesBeckman PDFElsiana LaurenciaNo ratings yet
- Comparison Between Folin-Ciocalteu and Prussian Blue Assays To Estimate The Total Phenolic Content of Juices and Teas Using 96-Well MicroplatesDocument7 pagesComparison Between Folin-Ciocalteu and Prussian Blue Assays To Estimate The Total Phenolic Content of Juices and Teas Using 96-Well MicroplatesIsnaeni RachmawatiNo ratings yet
- SpectrophotometryDocument19 pagesSpectrophotometryabhinav_ramana100% (1)
- Determination of Sulphur Content of Un-Alloyed and Low-Alloyed Steel by Icp-Aes Spectrometry Using Wet Chemical Sample PreparationDocument8 pagesDetermination of Sulphur Content of Un-Alloyed and Low-Alloyed Steel by Icp-Aes Spectrometry Using Wet Chemical Sample PreparationngobaochanNo ratings yet
- Zhu Et Al., 2022 - Biogenic T&ODocument17 pagesZhu Et Al., 2022 - Biogenic T&OSusheeraNo ratings yet
- USP-NF 1467 Residual Solvents-Verification of Compendial Procedures and Validation of Alternative ProceduresDocument5 pagesUSP-NF 1467 Residual Solvents-Verification of Compendial Procedures and Validation of Alternative ProceduresAbsheen ZamanNo ratings yet
- Chapter 15 QDocument26 pagesChapter 15 QAbdelfattah Mohamed OufNo ratings yet
- Fortified Rice Fssai Approved MethodDocument25 pagesFortified Rice Fssai Approved MethodIFS FCI GURUGRAMNo ratings yet
- Fulltext 4 PDFDocument4 pagesFulltext 4 PDFSubhadip Banerjee0% (1)
- Determination of Lead in Wax Crayon Using FaasDocument38 pagesDetermination of Lead in Wax Crayon Using FaasKassimNo ratings yet
- End Group 2Document22 pagesEnd Group 2Sabha Khalid shafiqNo ratings yet
- Insert TRIGL 0020767107322COIN V11 enDocument4 pagesInsert TRIGL 0020767107322COIN V11 entechlabNo ratings yet
- Comparison Between Three Chromatographic GC-ECD GCDocument8 pagesComparison Between Three Chromatographic GC-ECD GCGerónimo PerazzoNo ratings yet
- 5990-8685en Appnote 4100mp-Aes FoodsDocument6 pages5990-8685en Appnote 4100mp-Aes FoodsMackinder LijarzaNo ratings yet
- s00764 023 00230 7Document9 pagess00764 023 00230 7Artem KulikovNo ratings yet
- EDTAin CosmeticsDocument10 pagesEDTAin CosmeticsHammam HafidzurahmanNo ratings yet
- Quality Analysis of Virgin Olive Oils - : Application NoteDocument8 pagesQuality Analysis of Virgin Olive Oils - : Application NoteGiang Nguyễn Thị HươngNo ratings yet
- Spectrophotometric Method For The Determination of Amikacin in Pure and Pharmaceutical Dosage FormDocument5 pagesSpectrophotometric Method For The Determination of Amikacin in Pure and Pharmaceutical Dosage FormAshraf HamedNo ratings yet
- Insert CRP4 0108057591190c503 V5 enDocument4 pagesInsert CRP4 0108057591190c503 V5 enVegha NedyaNo ratings yet
- The Application of Atomic Absorption SpectrometryDocument10 pagesThe Application of Atomic Absorption SpectrometryAzizah AmaliyahNo ratings yet
- Method Oia-1677-09 2010Document28 pagesMethod Oia-1677-09 2010sergioalex1430No ratings yet
- 45095641Document12 pages45095641Pataki SandorNo ratings yet
- Dissolved Oxygen MeasurementDocument5 pagesDissolved Oxygen Measurementraju1559405No ratings yet
- Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics 7th Edition Burtis Test BankDocument8 pagesTietz Fundamentals of Clinical Chemistry and Molecular Diagnostics 7th Edition Burtis Test BankRicardoCarsonnbgda94% (17)
- 3.2. S.4.3.4 Validation of The GC Method For Determination of The Residual Solvents (Methanol, Ethanol) of Ofloxacin by GCDocument19 pages3.2. S.4.3.4 Validation of The GC Method For Determination of The Residual Solvents (Methanol, Ethanol) of Ofloxacin by GCRaul JimenezNo ratings yet
- Characteristic Concentration vs. Detection LimitDocument4 pagesCharacteristic Concentration vs. Detection LimitJorge Luis Bautista SantosNo ratings yet
- Week - 3 - Assignment and SolutionDocument10 pagesWeek - 3 - Assignment and SolutionAmith SharmaNo ratings yet