Professional Documents
Culture Documents
Infinosis T3-In017702 en
Uploaded by
Meditech visionbdOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Infinosis T3-In017702 en
Uploaded by
Meditech visionbdCopyright:
Available Formats
IN0177022105V1
POC T3 infinosis™
Triiodothyronine
REF: IN017702 25 tests • Don’t use Test Cassette if its Lot does not match with ID Chip that is inserted Step 5: Testing
onto the instrument. Put the Test Cassette on the operation platform. 15 minutes later, insert the Test
Intended use • The Infinosis™ T3 assay should be used with Infinosis™ 2020 FIA analyzer. Cassette onto the Cassette Holder and click “Test”. The result will show in the
The Infinosis™ T3 is an immunofluorescence-based lateral flow assay for the in • The tests should be applied by professionally trained staff working in certified display and print out when click “Print”.
vitro quantitative determination of triiodothyronine (total T3) in human serum or laboratories at some remove from the patient and clinic at which the sample is
plasma. taken by qualified medical personnel. Please refer to the Infinosis™ 2020 FIA analyzer Operation Manual for details.
• Infinosis™ T3 assay is single use only. Do not re-use it.
Summary1-7 • The Test Cassette and Analyzer should be used away from vibration and Limitations - interference
Triiodothyronine (T3) is the hormone principally responsible for the development magnetic field. During normal usage, the Test Cassette may introduce minute • The assay is unaffected by icterus (bilirubin < 600 μmol/L or < 35 mg/dL),
of the effects of the thyroid hormones on the various target organs. T3 (3,5,3’- vibration, which should be regarded as normal. hemolysis (Hb < 0.559 mmol/L or < 0.9 g/dL), lipemia (Intralipid < 1200 mg/dL),
triiodothyronine) is mainly formed extrathyroidally, particularly in the liver, by • Use separate clean pipette tips and detector buffer vials for different specimens. and biotin < 94 nmol/L or < 23 ng/mL.
enzymatic 5’-deiodination of T4. Accordingly, the T3 concentration in serum is The pipette tips and detector buffer vials should be used for one specimen only. • Criterion: Recovery within ± 10 % of initial value.
more a reflection of the functional state of the peripheral tissue than the secretory • Do not smoke, eat, or drink in areas in which specimens or kit reagents are • Heterophilic antibodies and rheumatoid factors in samples may interfere with
performance of the thyroid gland.
handled. test results. Heterophilic antibodies in human serum can react with reagent
A reduction in the conversion of T4 to T3 results in a decrease in
• Blood specimens, used test cassettes, pipette tips and detector buffer vials are immunoglobulins, interfering with in vitro immunoassays. Patients routinely
the T3 concentration. It occurs under the influence of medicaments such as potentially infectious. Proper laboratory safety techniques, handing and exposed to animals or animal serum products can be prone to this interference
propanolol, glucocorticoids or amiodarone and in severe non-thyroidal illness disposal methods should be followed in accordance with standard procedures and anomalous values may be observed. Additional information may be
(NTI), and is referred to as “low T3 syndrome”. As with T4, over 99 % of T3 is and relevant regulations observed by microbiological hazard materials. required for diagnosis. This kind of samples is not suitable to be tested by this
bound to transport proteins. However, the affinity of T3 to them is around 10-fold • The results should be interpreted by the physician along with clinical findings assay.
lower. and other laboratory test results. • Performance of this test has not been established with neonatal samples.
The determination of T3 is utilized in the diagnosis of T3-hyperthyroidism, the • The test will be applied on a routine basis and not in emergency situations. • Serum T3 concentration is dependent upon a multiplicity of factors:
detection of early stages of hyperthyroidism and for indicating a diagnosis of hypothalamus gland function and its regulation, TBG concentration, and the
thyrotoxicosis factitia. Storage and stability binding of T3 to TBG. Thus, total T3 concentration alone is not sufficient to
• Store the sample buffer at 4-30°C. The buffer is stable up to 24 months. assess clinical status.
Test principle • Store Infinosis™ test cassette at 4-30°C, shelf life is up to 24 months. • A decrease in total T3 values is found with protein-wasting diseases, certain
• Test cassette should be used within 1 hour after opening the pack. liver diseases and administration of testosterone, diphenylhy- dantoin or
Competitive principle. Total duration of assay: 25 minutes
salicylates. A table of interfering drugs and conditions, which affect total T3
Sample is added to the sample well of the test, the fluorescence-labeled detector
Specimen collection and preparation values, has been compiled by the Journal of the American Association of
T3 antibodies bind to T3 antigens in blood specimen and form immune
Clinical Chemists.
complexes. As the complexes migrate on the nitrocellulose matrix by capillary • The test can be performed with either serum or plasma.
action, it can’t be captured by T3 antigens that have been immobilized on test • Collect serum samples in accordance with correct medical practices.
strip, otherwise the excess unbound fluorescence-labeled detector T3 antibodies • Using standard phlebotomy procedure, collect a venipuncture whole blood
Limits and ranges
are captured. Thus the more T3 in blood, the less unbound fluorescence-labeled specimen using a blood collection tube. If collecting plasma use a blood Measuring range
antibodies accumulated on test strip. Signal intensity of detector T3 antibodies collection tube containing suitable anticoagulant (EDTA recommended). 0.45-6.0 ng/mL (defined by the lower detection limit and the maximum of the
reflect the amount of antigens and are processed in the Infinosis™ FIA system to master curve). Values below the detection limit are reported as < 0.45 ng/mL.
• Separate the serum/plasma from blood as soon as possible to avoid hemolysis.
determine the T3 concentration in blood. Values above the measuring range are reported as > 6.0 ng/mL
• Test should be performed immediately after the specimens have been collected.
Do not leave the specimens at room temperature for prolonged periods.
Reagents Specimens may be stored at 2-8°C for up to 3 days. For long-term storage, Lower limits of measurement
specimens should be kept below -20°C. Lower detection limit
Materials provided
Lower detection limit: 0.4 ng/mL
• Test cassette, 25 pcs, individually packaged The detection limit represents the lowest analyte level that can be distinguished
• ID chip, 1 pcs Quality control
from zero. It is calculated as the value lying two standard deviations above that of
• Sample buffer A, 1 vial, 2 mL Each Infinosis™ T3 test cassette contains internal control that satisfies routing
the lowest standard (master calibrator, standard 1 + 2 SD, repeatability study, n =
• Sample buffer B, 1 vial, 1 mL quality control requirements. This internal control is performed each time a patient
21).
• Centrifuge tube, 25 sample is tested. This control indicates that the test cartridge was inserted and
• IFU, 1 copy read properly by Infinosis™ 2020 FIA analyzer. An invalid result from the internal
Expected values
control causes an error message on Infinosis™ 2020 FIA analyzer indicating that
Materials required (but not provided) the test should be repeated. 0.8-2.11 ng/mL
These values correspond to the 2.5th and 97.5th percentiles of results obtained
• TSH control (Infinosis™ control is recommended)
from a total of 352 healthy test subjects examined.
• Infinosis™ 2020 FIA analyzer Test procedure
We have not studied the reference intervals in children, adolescents and pregnant
• Transfer pipette set (100 μL size) Refer to Infinosis™ 2020 FIA analyzer Operation Manual for the complete
women.
• Centrifuge (for plasma and serum only) instructions on use of the test. The test should be operated in room temperature.
Each laboratory should investigate the transferability of the expected values to its
• Timer Step 1: Preparation own patient population and if necessary determine its own reference ranges.
Check/insert ID Chip into the analyzer.
Precautions and warnings
Step 2: Sampling Specific performance data
• For in vitro diagnostic use only. Add 80 μL of Sample buffer A, 30 μL of Sample buffer B, and 20 μL of plasma/ Representative performance data are given below. Results obtained in individual
• Carefully follow the instructions and procedures described in this instructions serum to the Centrifuge tube.
before testing. laboratories may differ.
• The test cassette should remain in its original sealed pouch until ready to use. Step 3: Mixing
Do not use it if the pouch is damaged or the seal is broken. Mix the specimen well with buffer A and B for 10 minutes at room temperature Precision
• Do not use reagents beyond the labeled expiry date. (18-25℃) Intra-assay
• Do not mix or use components from kits with different Lots. Step 4: Loading Determined by using 10 tests in the same batch to test with T3 control, CV ≤ 15%
Add 80 μL of sample mixture and load it onto the sample well of the test cassette. Inter-assay
202105 V1 English 1/2 Infinosis™ T3/IFU
IN0177022105V1
POC T3 infinosis™
Triiodothyronine
Determined by using 3 tests in 3 random and continuous batches to test with T3
control, CV ≤ 20%
Method comparison
A comparison of the Infinosis™ T3 assay (y) with the Roche Elecsys T3 (x) using
diasino
clinical samples gave the following correlation: DiaSino Laboratories Co., Ltd
No.68, Jingnansi Road, National Eco & Tech Development Area
Number of samples measured: 312 Zhengzhou, China. 450000
Linear regression Technical Support: ts@diasino.com
www.diasino.com
y = 1.0309X - 0.2539
r = 0.9855
Analytical specificity
For the antibody derivative used, the following cross-reactivities were found: D-T3
100 %; L-T4 < 0.18 %; D-T4 < 0.18 %; L-rT3 < 0.05 %; L-T2 < 0.9 %.
References
1. Wheeler MH, Lazarus JH. Diseases of the Thyroid. London, Glasgow,
Weinheim, New York, Tokyo, Melbourne, Madras: Chapman and Hall Medical,
1994:107-115.
2. Pfannenstiel P, Saller B. Schilddrüsenkrankheiten Diagnose und Therapie.
Berliner Medizinische Verlagsanstalt GmbH, 1995;2:30-32,60-62.
3. Fisher DA. Physiological variations in thyroid hormones; physiological and
pathophysiological considerations. Clinical Chemistry 1996;42:135-139.
4. Tietz NW. Clinical Guide To Laboratory Tests. 3rd ed. Philadelphia, Pa: WB
Saunders Co, 1995:612.
5. Surks MI, Chopra IJ, Mariash CN, Nicoloff JT, Solomon DH. American Thyroid
Association guidelines for use of laboratory tests in thyroid disorders. JAMA
1990;63:1529-1532.
6. Becker DV, Bigos ST, Gaitan E, Morris JC, Rallison ML, Spencer CA, et al.
Optimal use of blood tests for assessment of thyroid
function (letter). JAMA 1993;269:273.
7. Klee GG. Clinical usage recommendations and analytic performance goals for
total and free triiodothyronine measurements. Clinical Chemistry
1996;42:155-159.
In vitro diagnostic Refer to instruction
use for use
Expiry date Manufacturing date
Batch number Test per kit
Catalog number Do not re-use
Store at 4-30℃ Manufacturer
202105 V1 English 2/2 Infinosis™ T3/IFU
You might also like
- Infinosis T4 IN017703 CEDocument2 pagesInfinosis T4 IN017703 CEaillNo ratings yet
- Infinosis Troponin I IN047701 enDocument2 pagesInfinosis Troponin I IN047701 enMeditech visionbdNo ratings yet
- Package Insert - 07204 - I - en - 30403 T3 PDFDocument5 pagesPackage Insert - 07204 - I - en - 30403 T3 PDFadybaila4680No ratings yet
- Free T3 - IMMULITEDocument36 pagesFree T3 - IMMULITEEdgar GalvánNo ratings yet
- T4 Reactiv ChemiluminiscentaDocument5 pagesT4 Reactiv ChemiluminiscentaLidia NarbNo ratings yet
- EnglishDocument3 pagesEnglishArnaz AdisaputraNo ratings yet
- 25 Tt4hu E01 PDFDocument9 pages25 Tt4hu E01 PDFDzejmenMerlinaNo ratings yet
- Lab Policies Free Thyroxine FT4 Cobas E601 Lab 4045Document4 pagesLab Policies Free Thyroxine FT4 Cobas E601 Lab 4045TohăneanR.RomeliaNo ratings yet
- Enzyme Immunoassay For The Quantitative Determination of Triiodothyronine (T3) in Human SerumDocument10 pagesEnzyme Immunoassay For The Quantitative Determination of Triiodothyronine (T3) in Human SerumcitrahdynNo ratings yet
- Infinosis PCT IN057701 enDocument2 pagesInfinosis PCT IN057701 enMeditech visionbdNo ratings yet
- Free Thyroxine (FT4) Test Kit (Homogeneous Chemiluminescence Immunoassay) Instruction For Use A0Document2 pagesFree Thyroxine (FT4) Test Kit (Homogeneous Chemiluminescence Immunoassay) Instruction For Use A0Asesoria TecnicaNo ratings yet
- Total T3: For Use On The IMMULITE and Immulite 1000 SystemsDocument31 pagesTotal T3: For Use On The IMMULITE and Immulite 1000 SystemsEdgar Galván0% (1)
- Can-Fte-260 - Free TestosteroneDocument2 pagesCan-Fte-260 - Free TestosteronevijayaNo ratings yet
- Thyroid Stimulating Hormone (TSH) CLIADocument2 pagesThyroid Stimulating Hormone (TSH) CLIAp11.sethiaNo ratings yet
- T UptakeDocument3 pagesT UptakeModestus100% (1)
- Free Triiodothyronine (Ft3) Enzyme Immunoassay Test Kit Catalog Number: BC-1006Document3 pagesFree Triiodothyronine (Ft3) Enzyme Immunoassay Test Kit Catalog Number: BC-1006Juan SánchezNo ratings yet
- T4Document26 pagesT4Florea RodicaNo ratings yet
- Anti-Thyroid Peroxidase Antibody (Anti-TPO) CLIA: 2 X 50 Test 52025070Document2 pagesAnti-Thyroid Peroxidase Antibody (Anti-TPO) CLIA: 2 X 50 Test 52025070p11.sethiaNo ratings yet
- Infinosis LH IN027703 enDocument2 pagesInfinosis LH IN027703 enMeditech visionbdNo ratings yet
- Totalt3 ArcDocument6 pagesTotalt3 ArcTanveerNo ratings yet
- Free THYROXINE (fT4) : Enzyme Immunoassay Test Kit Catalog Number: 10306Document4 pagesFree THYROXINE (fT4) : Enzyme Immunoassay Test Kit Catalog Number: 10306Yousra ZeidanNo ratings yet
- Inserto T3Document2 pagesInserto T3cesiahdez0% (2)
- 30 462 Vidas Anti-Tg (ATG) : Summary and ExplanationDocument8 pages30 462 Vidas Anti-Tg (ATG) : Summary and ExplanationHaider AlmothaferNo ratings yet
- Reference: Maglumi TG (Clia)Document4 pagesReference: Maglumi TG (Clia)Lidia NarbNo ratings yet
- Anti - TG 30462Document8 pagesAnti - TG 30462armada thamNo ratings yet
- FT3Document26 pagesFT3Florea RodicaNo ratings yet
- Prothrombin Time SOPDocument3 pagesProthrombin Time SOPGamal Eldien FathiNo ratings yet
- Intended Use: Ichroma™ T4 Is A Fluorescence Immunoassay (FIA) For TheDocument4 pagesIntended Use: Ichroma™ T4 Is A Fluorescence Immunoassay (FIA) For TheRizoreNo ratings yet
- Snibe Maglumi Ft3 CliaDocument4 pagesSnibe Maglumi Ft3 CliaEsraa MahmoudNo ratings yet
- Intended Use: Ichroma™ TSH Is A Fluorescence Immunoassay (FIA) ForDocument4 pagesIntended Use: Ichroma™ TSH Is A Fluorescence Immunoassay (FIA) ForFathi MesoNo ratings yet
- T3 Rapid Quantitative Test COA - F2311630AADDocument1 pageT3 Rapid Quantitative Test COA - F2311630AADg64bt8rqdwNo ratings yet
- Ft3 IflashDocument4 pagesFt3 IflashNIGHT tubeNo ratings yet
- Free T4 - IMMUNOLITE 2000Document36 pagesFree T4 - IMMUNOLITE 2000pyx5pjqqx4No ratings yet
- TG II BiotinDocument5 pagesTG II BiotinNazaqat FarooqNo ratings yet
- Enzyme Immunoassay Test Kit Catalog Number: 10304: Thyroid Stimulating Hormone (TSH)Document2 pagesEnzyme Immunoassay Test Kit Catalog Number: 10304: Thyroid Stimulating Hormone (TSH)petertrungNo ratings yet
- Enzyme: Tiiiitiinllll - .Document4 pagesEnzyme: Tiiiitiinllll - .Ruchika NaglaNo ratings yet
- Infinosis HCG - IN027702 - enDocument2 pagesInfinosis HCG - IN027702 - enMeditech visionbdNo ratings yet
- Maglumi Ft3 (Clia) : Intended UseDocument4 pagesMaglumi Ft3 (Clia) : Intended UseYaser MNo ratings yet
- Cardiac Troponin TDocument2 pagesCardiac Troponin TModestusNo ratings yet
- Tn-I Plus: Warnings and PrecautionsDocument5 pagesTn-I Plus: Warnings and PrecautionsAniket DubeyNo ratings yet
- Total T4: For Use On The IMMULITE® and IMMULITE® 1000 SystemsDocument34 pagesTotal T4: For Use On The IMMULITE® and IMMULITE® 1000 SystemsEdgar GalvánNo ratings yet
- Package Insert - FT3 PDFDocument6 pagesPackage Insert - FT3 PDFKadek Ayang Cendana Prahayu0% (1)
- TSH Rapid Quantitative Test COA-F22017702ADDocument1 pageTSH Rapid Quantitative Test COA-F22017702ADg64bt8rqdwNo ratings yet
- iFlash T3 Immunoassay Analyzer Reagent KitDocument4 pagesiFlash T3 Immunoassay Analyzer Reagent KitNIGHT tubeNo ratings yet
- Trichomonas Rapid Test: CLIA Complexity: WaivedDocument8 pagesTrichomonas Rapid Test: CLIA Complexity: WaivedRabecca TobingNo ratings yet
- el-t3Document2 pagesel-t3Nghi NguyenNo ratings yet
- Insert - Elecsys T3.ms - 11731360122.v26.enDocument4 pagesInsert - Elecsys T3.ms - 11731360122.v26.enGuneyden Guneyden100% (3)
- (INS-TN-EN) Tn-I - Rev.14 - 160720Document3 pages(INS-TN-EN) Tn-I - Rev.14 - 160720nam7124119No ratings yet
- PT Calcium Package InsertDocument2 pagesPT Calcium Package InsertManikanta Sai KumarNo ratings yet
- Elisa Troponin TDocument15 pagesElisa Troponin Tfadil fadlanNo ratings yet
- Access TSH (3rd IS) Instructions For Use ThyrotropinDocument13 pagesAccess TSH (3rd IS) Instructions For Use ThyrotropincarineNo ratings yet
- PT, Aptt, TTDocument44 pagesPT, Aptt, TTswaraj sharmaNo ratings yet
- INS PP EN Ichroma PCT Plus - Rev.03 - 181112Document4 pagesINS PP EN Ichroma PCT Plus - Rev.03 - 181112Bilqist NabillaNo ratings yet
- Ggt-Drug-Ogtt-24hr (Answer To Sample Questions)Document4 pagesGgt-Drug-Ogtt-24hr (Answer To Sample Questions)maja.amora.swuNo ratings yet
- Kit Insert NtprobnpDocument12 pagesKit Insert NtprobnpRidho Kurnia IINo ratings yet
- A TpoDocument4 pagesA TpoJimboreanu György PaulaNo ratings yet
- MAN0028592 PierceRapidGelClotEndotoxinAssayKits UGDocument2 pagesMAN0028592 PierceRapidGelClotEndotoxinAssayKits UGanalista.microbiologiaNo ratings yet
- Elecsys FT3Document4 pagesElecsys FT3Arnaz AdisaputraNo ratings yet
- Infinosis PRL IN027705 enDocument2 pagesInfinosis PRL IN027705 enMeditech visionbdNo ratings yet
- Infinosis™ Presentation - 18062021V1Document20 pagesInfinosis™ Presentation - 18062021V1Meditech visionbdNo ratings yet
- Infinosis HCG - IN027702 - enDocument2 pagesInfinosis HCG - IN027702 - enMeditech visionbdNo ratings yet
- Infinosis NT-proBNP IN047705 enDocument2 pagesInfinosis NT-proBNP IN047705 enMeditech visionbdNo ratings yet
- Infinosis TSH-In017701 enDocument2 pagesInfinosis TSH-In017701 enMeditech visionbdNo ratings yet
- Infinosis MAU IN067703 enDocument2 pagesInfinosis MAU IN067703 enMeditech visionbdNo ratings yet
- Infinosis T4-In017703 enDocument2 pagesInfinosis T4-In017703 enMeditech visionbdNo ratings yet
- Infinosis Total IgE IN067705 enDocument2 pagesInfinosis Total IgE IN067705 enMeditech visionbdNo ratings yet
- Infinosis IL-6 IN057704 enDocument2 pagesInfinosis IL-6 IN057704 enMeditech visionbdNo ratings yet
- Infinosis LH IN027703 enDocument2 pagesInfinosis LH IN027703 enMeditech visionbdNo ratings yet
- Price Quotation For Pathological Reagents. Sir,: (IFCC) (IFCC) (IFCC)Document1 pagePrice Quotation For Pathological Reagents. Sir,: (IFCC) (IFCC) (IFCC)Meditech visionbdNo ratings yet
- Infinosis HbA1c IN067701 enDocument2 pagesInfinosis HbA1c IN067701 enMeditech visionbdNo ratings yet
- Infinosis Myo IN047702 enDocument2 pagesInfinosis Myo IN047702 enMeditech visionbdNo ratings yet
- Infinosis PCT IN057701 enDocument2 pagesInfinosis PCT IN057701 enMeditech visionbdNo ratings yet
- Infinosis CRP IN057702 enDocument2 pagesInfinosis CRP IN057702 enMeditech visionbdNo ratings yet
- Infinosis FSH IN027704 enDocument2 pagesInfinosis FSH IN027704 enMeditech visionbdNo ratings yet
- Link 3 TechnologyDocument1 pageLink 3 TechnologyMeditech visionbdNo ratings yet
- New Microsoft Office Word DocumentDocument3 pagesNew Microsoft Office Word DocumentMeditech visionbdNo ratings yet
- Qvî/Qvîxi BVG T Ivj Bs Köbx WelqDocument13 pagesQvî/Qvîxi BVG T Ivj Bs Köbx WelqMeditech visionbdNo ratings yet
- Biochemistry Chema, Italy: (IFCC) (IFCC) (IFCC)Document2 pagesBiochemistry Chema, Italy: (IFCC) (IFCC) (IFCC)Meditech visionbdNo ratings yet
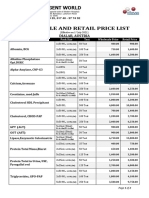
- Price List Wholesale and Retail (June-2021)Document2 pagesPrice List Wholesale and Retail (June-2021)Meditech visionbdNo ratings yet
- Welq T KV Ri Avb ': Qvî/Qvîxi BVG T Gvzvi BVG T Wczvi BVG T Ivj Bs TDocument19 pagesWelq T KV Ri Avb ': Qvî/Qvîxi BVG T Gvzvi BVG T Wczvi BVG T Ivj Bs TMeditech visionbdNo ratings yet
- Infinosis CK-MB - IN047703 - enDocument2 pagesInfinosis CK-MB - IN047703 - enMeditech visionbdNo ratings yet
- 7915 01933902846 101103689110 7be4cszp90Document1 page7915 01933902846 101103689110 7be4cszp90Meditech visionbdNo ratings yet
- Price Quotation For Following Lab AccessoriesDocument1 pagePrice Quotation For Following Lab AccessoriesMeditech visionbdNo ratings yet
- Price Quotation For Following Lab AccessoriesDocument1 pagePrice Quotation For Following Lab AccessoriesMeditech visionbdNo ratings yet
- M T Jute Trading: Count & Quality: 10/1 LBS 1 PLY HessianDocument4 pagesM T Jute Trading: Count & Quality: 10/1 LBS 1 PLY HessianMeditech visionbdNo ratings yet
- Amylase enDocument1 pageAmylase enRakib Hossain 3A-159No ratings yet
- The Role of Technology in AgricultureDocument35 pagesThe Role of Technology in AgricultureDivyesh ThumarNo ratings yet
- SECTION 03380 Post-Tensioned Concrete Rev 1Document30 pagesSECTION 03380 Post-Tensioned Concrete Rev 1Abdalrahman AntariNo ratings yet
- MMDST PDFDocument50 pagesMMDST PDFChristopher OlipasNo ratings yet
- Independent Study Report # 1Document75 pagesIndependent Study Report # 1Sunny DubeyNo ratings yet
- A4 RelationshipgridDocument32 pagesA4 RelationshipgridjamesNo ratings yet
- ANPATH1 ReviewerDocument17 pagesANPATH1 ReviewerRashid DayaoNo ratings yet
- Dahong PalayDocument2 pagesDahong PalayAngela MontenegroNo ratings yet
- Chery Amulet 1,6 Engine Service ManualDocument76 pagesChery Amulet 1,6 Engine Service ManualG x HxhNo ratings yet
- PackageCare Maintenance ChecklistDocument1 pagePackageCare Maintenance ChecklistBùi ViệtNo ratings yet
- Indian Medical Tourism Industry: A Pathway For The Healthy Future of IndiaDocument13 pagesIndian Medical Tourism Industry: A Pathway For The Healthy Future of IndiaPranjal MaluNo ratings yet
- Tutorial Sheet 4Document2 pagesTutorial Sheet 4Syed YousufuddinNo ratings yet
- Zollinger-Ellison Syndrome (Gastrinoma)Document15 pagesZollinger-Ellison Syndrome (Gastrinoma)Huy QuangNo ratings yet
- Healthy Voice: by Dan VascDocument22 pagesHealthy Voice: by Dan VascscoutjohnyNo ratings yet
- Implementation of Swachh Bharat in MysoreDocument13 pagesImplementation of Swachh Bharat in MysoreDipyaman ChoudhuryNo ratings yet
- Economic Case for Immobilizing EnzymesDocument25 pagesEconomic Case for Immobilizing EnzymesNikki ChauhanNo ratings yet
- Lubricants: Chapter - 5 Lubricants and LubricationDocument5 pagesLubricants: Chapter - 5 Lubricants and LubricationMalaika AzeemNo ratings yet
- BONDING: Understanding Chemical AttractionsDocument201 pagesBONDING: Understanding Chemical AttractionsKhaled OsmanNo ratings yet
- Population Ecology: Aecc-I +3 1 YearDocument32 pagesPopulation Ecology: Aecc-I +3 1 YearAnita kumari SahuNo ratings yet
- May 4-22 Culinary 1 Lesson Plans PDFDocument4 pagesMay 4-22 Culinary 1 Lesson Plans PDFesefsdfsfNo ratings yet
- Track A Beast ManualDocument21 pagesTrack A Beast ManualboltojamNo ratings yet
- Sprocket Asa 180Document1 pageSprocket Asa 180jhampolrosalesNo ratings yet
- A Project Report: Maya Engineering WorksDocument2 pagesA Project Report: Maya Engineering WorksVishwendra SinghNo ratings yet
- Rational Choice TheoryDocument6 pagesRational Choice TheoryMaria Theresa HerbolingoNo ratings yet
- MKT421 New Product Development PDFDocument26 pagesMKT421 New Product Development PDFNass AzwadiNo ratings yet
- GORUCK Heavy - 6-week training program overviewDocument4 pagesGORUCK Heavy - 6-week training program overviewJohn Rohrer100% (2)
- Septic Abortion PDFDocument4 pagesSeptic Abortion PDFmariachrismayaniNo ratings yet
- Market ReseachDocument3 pagesMarket ReseachSam Cy PuyalesNo ratings yet
- Agriculture Class-8Document67 pagesAgriculture Class-8Sahej100% (2)
- Plumbing-Water-System-Review (3B) PDFDocument22 pagesPlumbing-Water-System-Review (3B) PDFJhyneJazarenoAtutuboNo ratings yet
- S# Isin CFI Code (As Per New ISO) Security Name Security Symbol Sector Name Security Type StatusDocument25 pagesS# Isin CFI Code (As Per New ISO) Security Name Security Symbol Sector Name Security Type StatusahmedalishNo ratings yet