Professional Documents
Culture Documents
Infinosis Total IgE IN067705 en
Uploaded by
Meditech visionbdOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Infinosis Total IgE IN067705 en
Uploaded by
Meditech visionbdCopyright:
Available Formats
IN0677052105V1
POC Total IgE infinosis™
Immunoglobulin E
REF: IN067705 25 tests • Transfer pipette set (100 μL size) Each Infinosis™ Total IgE test cassette contains internal control that satisfies
• Alcohol pads routing quality control requirements. This internal control is performed each time a
Intended use • Centrifuge (for plasma and serum only) patient sample is tested. This control indicates that the test cartridge was inserted
The Infinosis™ Total IgE is an immunofluorescence-based lateral flow assay for • Timer and read properly by Infinosis™ 2020 FIA analyzer. An invalid result from the
the in vitro quantitative determination of Immunoglobulin E in human whole blood, internal control causes an error message on Infinosis™ 2020 FIA analyzer
serum or plasma. Precautions and warnings indicating that the test should be repeated.
• For in vitro diagnostic use only.
Summary1-9 • Carefully follow the instructions and procedures described in this instructions Test procedure
Indications: allergic diseases, helminthiasis, eczematous or non eczematous before testing. Refer to Infinosis™ 2020 FIA analyzer Operation Manual for the complete
dermatitis, IgE myeloma, etc. Diagnosis of allergic reactions and atopic diseases: • The test cassette should remain in its original sealed pouch until ready to use. instructions on use of the test. The test should be operated in room temperature.
in addition to skin and provocation tests and detection of specific IgE, the Do not use it if the pouch is damaged or the seal is broken. Step 1: Preparation
diagnosis of allergic diseasesalso includes detection of total IgE level. However, • Do not use reagents beyond the labeled expiry date. Check/insert ID Chip into the analyzer.
allergic reactions were not always accompanied by an increase in total IgE levels • Do not mix or use components from kits with different Lots.
Don’t use Test Cassette if its Lot does not match with ID Chip that is inserted Step 2: Sampling
(adults > 100 IU/ml). On the contrary, low levels of IgE (adult < 25 IU/ml) can not •
onto the instrument. Add 20μL of whole blood, serum or plasma to the buffer tube.
rule out allergic reaction. Through long-term desensitization treatment and
keeping away from allergens, the total IgE titer usually decreases.By measuring • The Infinosis™ Total IgE assay should be used with Infinosis™ 2020 FIA Step 3: Mixing
the total IgE level, allergic asthma and endogenous asthma, allergic rhinitis and analyzer. Mix the specimen well with buffer for 5-10 seconds by tapping or inverting the
vasomotor rhinitis, as well as infant atopic dermatitis and seborrheic dermatitis • The tests should be applied by professionally trained staff working in certified tube.
can be distinguished. High concentrations of IgE (thousands of IU/mL) were found laboratories at some remove from the patient and clinic at which the sample is Step 4: Loading
in patients with atopic dermatitis. Other allergic (and high IgE) diseases include taken by qualified medical personnel. Add 80 μL of sample mixture and load it onto the sample well of the test cassette.
acute recurrent or chronic urticaria, recurrent Quincke edema (angioneurotic • Infinosis™ Total IgE assay is single use only. Do not re-use it.
Step 5: Testing
edema), gastrointestinal intolerance, and rashes of unknown origin. Detection of • The Test Cassette and Analyzer should be used away from vibration and
Put the Test Cassette on the operation platform. 15 minutes later, insert the Test
total IgE can also be used in the differential diagnosis of pulmonary eosinophilic magnetic field. During normal usage, the Test Cassette may introduce minute
Cassette onto the Cassette Holder and click “Test”. The result will show in the
infiltration, allergic aspergillosis, exogenous allergic alveolitis (farmer's lung and vibration, which should be regarded as normal.
display and print out when click “Print”.
pigeon's lung) and church strau β syndrome.IgE in other diseases: non allergic • Use separate clean pipette tips and detector buffer vials for different specimens.
diseases with high IgE levels include various forms of helminthiasis, such as The pipette tips and detector buffer vials should be used for one specimen only. Please refer to the Infinosis™ 2020 FIA analyzer Operation Manual for details.
toxocariasis, ascariasis, schistosomiasis, hookworm disease, leishmaniasis and • Do not smoke, eat, or drink in areas in which specimens or kit reagents are
trichonematodiasis. However, no increase of IgE level was found in taeniasis and handled. Limitations - interference
enterobiasis. For most cases, after effective treatment, IgE level can be reduced • Blood specimens, used test cassettes, pipette tips and detector buffer vials are
• The assay is unaffected by icterus (bilirubin < 1112 μmol/L or < 65 mg/dL),
to normal range. High concentrations of IgE can be detected in the following potentially infectious. Proper laboratory safety techniques, handing and hemolysis (Hb < 0.31 mmol/L or < 0.5 g/dL), lipemia (Intralipid < 3300 mg/dL)
diseases: eczematous or non eczematous dermatitis, IgE myeloma, Acute disposal methods should be followed in accordance with standard procedures and biotin (< 205 nmol/L or < 50 ng/mL).
systemic lupus erythematosus (SLE), Graft versus host response, T cell defect and relevant regulations observed by microbiological hazard materials.
• Criterion: Recovery within ± 10 % of initial value.
(Wiskott Aldrich syndrome), second or third degree burns, Otorhinolaryngological • The results should be interpreted by the physician along with clinical findings
• Samples should not be taken from patients receiving therapy with high biotin
tumors, Liver disease (especially related to alcohol abuse), Late stage of AIDS and other laboratory test results. doses (i.e. > 5 mg/day) until at least 8 hours following the last biotin
(CD4 + T cells decreased significantly) IgE deficiency may occur in the following • The test will be applied on a routine basis and not in emergency situations. administration.
diseases: x-chromosome-related hypogammaglobulinemia, Severe combined • No interference was observed from rheumatoid factors up to a concentration of
immunodeficiency (SCID), and Pulmonary fibrosis disease. Storage and stability 2500 IU/mL.
• Store the Sample buffer at 4-30°C. The buffer is stable up to 24 months. • There is no high-dose hook effect at Total IgE concentrations of
• Store Infinosis™ test cassette at 4-30°C, shelf life is up to 24 months. up to 50000 μg/L (ng/mL).
Test principle • Test cassette should be used within 1 hour after opening the pack. • In vitro tests were performed on 19 commonly used pharmaceuticals.
Sandwich principle. Total duration of assay: 15 minutes • No interference with the assay was found.
Sample is added to the sample well of the test, then the fluorescence-labeled Specimen collection and preparation • Iron2+- and iron3+-ions at therapeutic concentrations do not interfere with the
detector anti-Total IgE antibody binds to Total IgE antigen in blood specimen. As The test can be performed with either serum or plasma or whole blood. DiaSino Total IgE assay.
the sample mixture migrates on the nitrocellulose matrix of test strip by capillary Whole Blood Collected by Venipuncture: • In rare cases, interference due to extremely high titers of antibodies to analyte-
action, the complexes of detector antibody and Total IgE are captured to anti-Total specific antibodies, streptavidin or ruthenium can occur. These effects are
• Using standard phlebotomy procedure, collect a venipuncture whole blood
IgE antibody that has been immobilized on test strip. specimen using a blood collection tube with suitable anticoagulant (EDTA minimized by suitable test design.
The more Total IgE antigen is in blood specimen, the more complexes are recommended) • For diagnostic purposes, the results should always be assessed in conjunction
accumulated on test strip. Signal intensity of fluorescence of detector antibody with the patient’s medical history, clinical examination and other findings.
• It is recommended that specimens should be tested immediately. Do not leave
reflects amount of Total IgE captured and Infinosis™ FIA analyzer shows Total IgE the specimens at room temperature for prolonged periods. If the specimens are
concentrations in blood specimen. The default results unit of Infinosis™ Total IgE not tested immediately, they may be stored at 2-8°C. Limits and ranges
test is displayed as x ng/mL from Infinosis™ FIA analyzer. • It’s not suitable to test the whole blood samples which have been stored at Measuring range
2-8°C for more than 2 days. 7.0-1000 IU/mL (defined by the lower detection limit and the maximum of the
Reagents Serum and Plasma: master curve). Values below the lower detection limit are reported as < 7.0 IU/mL.
Materials provided Values above the measuring range are reported as > 1000 IU/mL.
• Separate the serum/plasma from blood as soon as possible to avoid hemolysis.
• Test cassette, 25 pcs, individually packaged • Test should be performed immediately after the specimens have been collected.
• ID chip, 1 pcs • Do not leave the specimens at room temperature for prolonged periods. Lower limits of measurement
• Sample buffer, 25 vials Specimens may be stored at 2 - 8℃ for up to 3 days. For long-term storage, Lower detection limit
• IFU, 1 copy specimens should be kept below -20℃. Lower detection limit: 7.0 IU/mL
The detection limit represents the lowest analyte level that can be distinguished
Materials required (but not provided) from zero. It is calculated as the value lying two standard deviations above that of
• Infinosis™ 2020 FIA analyzer Quality control
202105 V1 English 1/2 Infinosis™ Total IgE/IFU
IN0677052105V1
POC Total IgE infinosis™
Immunoglobulin E
the lowest standard (master calibrator, standard 1 + 2 SD, repeatability study, n = 6. Barbee RA, Halonen M, Kaltenborn W, Lebowitz M, Burrows B. A longitudinal
21). study of serum IgE in a community cohort: Correlations with age, sex, smoking,
and atopic status. J Allergy Clin Immunol; 79: 919-927 (1987)
Expected values 7. Elkayam O, Tamir R, Pick AI, Wysenbeek A. Serum IgE concentrations, disease
Reference range activity, and atopic disorders in systemic lupus erythematosus. Allergy; 50:
94-96 (1995)
Age Normal range(IU/mL) 8. Yates VM, Kerr REI, Frier K, Cobb SJ, MacKie RM. Early diagnosis of infantile
Newborns < 1.4 seborrhoeic dermatitis and atopic dermatits – total and specific IgE levels.
British Journal of Dermatology; 108: 639-645 (1982)
1-6 months < 7.5
9. Vidal C, Quintela AG, Millán I, Gude F, Cuervas-Mons V. Serum IgE levels in
7-12 months < 13 liver cirrhosis. Contrasting results in alcoholic and non-alcoholic patients. Clin
1-5 years old < 58 Exp Allergy; 24: 540-548 (1994)
6-9 years old < 167
10-15 years old < 202
In vitro diagnostic Refer to instruction
> 16 years old < 97 use for use
Expected values may vary with age, sex, diet and geographical location. Each
laboratory should determine its own expected values as dictated by good
laboratory practice. Expiry date Manufacturing date
Specific performance data
Representative performance data are given below. Results obtained in individual Batch number Test per kit
laboratories may differ.
Precision Catalog number Do not re-use
Intra-assay
Determined by by using 10 replicates from same batch to test with 500 IU/mL
Total IgE control, CV ≤ 10%. Store between
Manufacturer
Inter-assay
4-30℃
Determined by using 3 replicates from random 3 continuous batches to test with
500 IU/mL Total IgE control, CV ≤ 15%.
Linearity
A serial concentration of Total IgE controls at 10 IU/mL, 20 IU/mL and 50 IU/mL,
100 IU/mL, 150 IU/mL, 200 IU/mL were tested, the Correlation Coefficient is r ≥
0.9912.
Method comparison
A comparison of the Infinosis™ Total IgE assay (y) with the Roche Elecsys Total
IgE assay (x) using 79 clinical samples gave the correlation: r=0.9512
diasino
References DiaSino Laboratories Co., Ltd
1. Klink M, Cline MG, Halonen M, Burrows B. Problems in definig norma limits for No.68, Jingnansi Road, National Eco & Tech Development Area
serum IgE. J Allergy Clin Immunol; 85: 440-444 (1990) Zhengzhou, China. 450000
2. Kerkhof M, Droste JHJ, de Monchy JGR, Schouten JP, Rijcken B.Distribution of Technical Support: ts@diasino.com
total serum IgE and specific IgE to commo aeroallergens by sex and age, and www.diasino.com
their relationship to each other in a random sample of the Duch general
population aged 20-70 years. Allergy; 51: 770-776 (1996)
3. Kerstjens HAM, Schouten JP, Brand PLP, Schoonbrood DFME, Sterk PJ,
Postma DS. Importance of total serum IgE for improvement in airways
hyperresponsiveness with inhaled corticosteroids in asthma and chronic
obstructive pulmonary disease. Am J Respir Crit Care Med;151: 360-368 (1995)
4. Omenaas E, Bakke P, Elsayed S, Hanoa R, Gulsvik A. Total and specific serum
IgE levels in adults: relationship to sex, age and environmental factors. Clin Exp
Allergy; 24: 530-539 (1994)
5. Secord EA, Kleiner GI, Auci Dl, Smith-Norowitz T, Chice S, Finkielstein A,
Nowakowski M, Fikrig S, Durkin H.G. IgE against HIV proteins in clinically
healthy children with HIV disease. J Allergy Clin Immunol; 98:979-984 (1996)
202105 V1 English 2/2 Infinosis™ Total IgE/IFU
You might also like
- Total Ige PDFDocument8 pagesTotal Ige PDFahmad khoirul anwarNo ratings yet
- 3gallergy Specific IgE Universal Kit Panel Allergen CLSI - IMMULITE 2000 Systems - Rev 3 DXDCM 09017fe9803bd60d-1571818976833Document6 pages3gallergy Specific IgE Universal Kit Panel Allergen CLSI - IMMULITE 2000 Systems - Rev 3 DXDCM 09017fe9803bd60d-1571818976833Pierre LavoisierNo ratings yet
- Vir-Elisa Toxo-Igg Avidity 7.: (UREA)Document3 pagesVir-Elisa Toxo-Igg Avidity 7.: (UREA)Aghnia Asy S.No ratings yet
- 3gallergy 2020 - Specific - IgE - Universal - Kit - IMMULITE - 2000 - Systems - Rev - 32 - DXDCM - 09017fe9804a6eb0-1598648757334Document49 pages3gallergy 2020 - Specific - IgE - Universal - Kit - IMMULITE - 2000 - Systems - Rev - 32 - DXDCM - 09017fe9804a6eb0-1598648757334Pierre LavoisierNo ratings yet
- Allergy Diagnostics and Treatment 2022Document44 pagesAllergy Diagnostics and Treatment 2022Rohan TejaNo ratings yet
- Total Ige Elisa KitDocument2 pagesTotal Ige Elisa KitvaniaNo ratings yet
- Diagnostics of AllergiesDocument20 pagesDiagnostics of AllergiesPaola ViñéNo ratings yet
- 3gallergy Specific IgE Universal Kit CLSI - IMMULITE 2000 Systems - Rev 34 DXDCM 09017fe9807b401b-1676058951631Document7 pages3gallergy Specific IgE Universal Kit CLSI - IMMULITE 2000 Systems - Rev 34 DXDCM 09017fe9807b401b-1676058951631Pierre LavoisierNo ratings yet
- Total IgEDocument2 pagesTotal IgEMaherNo ratings yet
- Session 7Document15 pagesSession 7madcalNo ratings yet
- Allergy Testing PDFDocument25 pagesAllergy Testing PDFRK MalhotraNo ratings yet
- IgE II.V9Document4 pagesIgE II.V9Abdullah ZobayerNo ratings yet
- IgA ARC CHEMDocument8 pagesIgA ARC CHEMbassam alharaziNo ratings yet
- Trend in Laboratory Diagnostic For AllergyDocument29 pagesTrend in Laboratory Diagnostic For Allergygonteng sadyogaNo ratings yet
- AlaTOP Allergy Screen OUS - IMMULITE 2000 Systems - Rev 21 DXDCM 09017fe98067cfcb-1645658153157Document28 pagesAlaTOP Allergy Screen OUS - IMMULITE 2000 Systems - Rev 21 DXDCM 09017fe98067cfcb-1645658153157Pierre LavoisierNo ratings yet
- Immune System TestsDocument19 pagesImmune System TestsRandy Ian F. GallegoNo ratings yet
- Im Ii MonoDocument3 pagesIm Ii MonoMaria SousaNo ratings yet
- Main Allergens Diagnosis of Allergic Diseases - Skin Prick Tests 2022Document52 pagesMain Allergens Diagnosis of Allergic Diseases - Skin Prick Tests 2022SePeHR SHNo ratings yet
- Appropriate Allergic Testing and InterpretationDocument40 pagesAppropriate Allergic Testing and InterpretationEllenNo ratings yet
- Igg Arc ChemDocument8 pagesIgg Arc Chembassam alharaziNo ratings yet
- Dengue Igm / Igg Rapid Test: Intended UseDocument4 pagesDengue Igm / Igg Rapid Test: Intended UseYvette TiongsonNo ratings yet
- A Rapid Test For Detection of Dengue FeverDocument2 pagesA Rapid Test For Detection of Dengue FeverYvette TiongsonNo ratings yet
- IGF-I - IMMULITE and IMMULITE 1000Document37 pagesIGF-I - IMMULITE and IMMULITE 1000LUIS DANIEL VAZQUEZ RAMIREZNo ratings yet
- HIV 12 Stat Pak Dipstick Product Packet EnglishDocument6 pagesHIV 12 Stat Pak Dipstick Product Packet EnglishSagkyNo ratings yet
- IgE Allergy Testing World Allergy Position PaperDocument50 pagesIgE Allergy Testing World Allergy Position PaperAlfred AlfredNo ratings yet
- Allergy Blood Testing: A Practical Guide For Clinicians: Roxana I. Siles, MDDocument16 pagesAllergy Blood Testing: A Practical Guide For Clinicians: Roxana I. Siles, MDSreejith BhattathiriNo ratings yet
- HAV IgG/IgM Test InstructionsDocument2 pagesHAV IgG/IgM Test InstructionsRuben DuranNo ratings yet
- Dengue IgG.igmDocument4 pagesDengue IgG.igmsatujuli23No ratings yet
- Igm Arc ChemDocument8 pagesIgm Arc Chembassam alharaziNo ratings yet
- Allergy Test: Faculty of Medicine Islamic University of MalangDocument37 pagesAllergy Test: Faculty of Medicine Islamic University of MalangDhonat FlashNo ratings yet
- selectiveigadeficiencyyes-131230034028-phpapp01 (1) (1) (1)Document27 pagesselectiveigadeficiencyyes-131230034028-phpapp01 (1) (1) (1)komalsaharan2001No ratings yet
- Insert - Elecsys Syphilis - Ms 07802960190.V3.EnDocument5 pagesInsert - Elecsys Syphilis - Ms 07802960190.V3.EnGuneyden GuneydenNo ratings yet
- COVID-19: Instructions For UseDocument2 pagesCOVID-19: Instructions For UseTheresia IlyanNo ratings yet
- Manual AdLeptospiraIgMIgGCardDocument2 pagesManual AdLeptospiraIgMIgGCardMatibar RahmanNo ratings yet
- Seminar WordDocument32 pagesSeminar Wordslmnkhn1988No ratings yet
- Antigen - Antibody Reactions Part 3Document25 pagesAntigen - Antibody Reactions Part 3Manas DixitNo ratings yet
- Hypersensitivity-NotesDocument7 pagesHypersensitivity-NotesShaii Whomewhat GuyguyonNo ratings yet
- Analizador GP Getein 1100 ManualDocument2 pagesAnalizador GP Getein 1100 Manualbiomedico international0% (1)
- HypersensitivityDocument59 pagesHypersensitivityGlenn SampayanNo ratings yet
- For Professional and in Vitro Diagnostic Use OnlyDocument6 pagesFor Professional and in Vitro Diagnostic Use OnlySheilla DifaNo ratings yet
- Pemeriksaan Penunjang 1. Skin Testing For Detection of Allergen-Specific IgeDocument7 pagesPemeriksaan Penunjang 1. Skin Testing For Detection of Allergen-Specific Igerosalia puspitajayaNo ratings yet
- Infinosis LH IN027703 enDocument2 pagesInfinosis LH IN027703 enMeditech visionbdNo ratings yet
- Skin TestDocument23 pagesSkin TestpraptiwiNo ratings yet
- Toxoplasma Gondii Igg Avidity Test: NovalisaDocument8 pagesToxoplasma Gondii Igg Avidity Test: NovalisaAghnia Asy S.No ratings yet
- Infinosis HbA1c IN067701 enDocument2 pagesInfinosis HbA1c IN067701 enMeditech visionbdNo ratings yet
- Detection of Allergen Specific IgE Antibody ResponsesDocument12 pagesDetection of Allergen Specific IgE Antibody ResponsesYulius DonyNo ratings yet
- Aminotransferaseserum Glutamic Oxaloacetic TransaminaseDocument3 pagesAminotransferaseserum Glutamic Oxaloacetic Transaminaserose_almonteNo ratings yet
- Diagnosis and Management of Allergic Conjunctivitis 2018Document10 pagesDiagnosis and Management of Allergic Conjunctivitis 2018Ricardo Robles AlfaroNo ratings yet
- Rubella Quantitative IgG - IMMULITE 2000 SystemsDocument40 pagesRubella Quantitative IgG - IMMULITE 2000 SystemsMaria Ruth Moreno VargasNo ratings yet
- Gribbles Heska Gvmar BF Nat 01542Document32 pagesGribbles Heska Gvmar BF Nat 01542Emna BouhajjaNo ratings yet
- Antigen: Canine Parvo VirusDocument2 pagesAntigen: Canine Parvo VirusPutrina SiregarNo ratings yet
- IVIGDocument7 pagesIVIGPeraNo ratings yet
- Covid-19 Igg/Igm Rapid Test Kit: Erick Esteban Paredes CedenoDocument2 pagesCovid-19 Igg/Igm Rapid Test Kit: Erick Esteban Paredes CedenoAlisonReinoso8No ratings yet
- Insert - Elecsys IgE II.04827031500.V12.enDocument4 pagesInsert - Elecsys IgE II.04827031500.V12.enRaj KumarNo ratings yet
- Allergic Rhinitis Slides 070926 PDFDocument57 pagesAllergic Rhinitis Slides 070926 PDFmhamad kabraNo ratings yet
- Allergy 2010Document7 pagesAllergy 2010HAOMSNo ratings yet
- ASCIA HP SPT Guide 2020Document22 pagesASCIA HP SPT Guide 2020Edy NoveryNo ratings yet
- ElisaDocument13 pagesElisaSHOBHIT KUMAR MUNANo ratings yet
- IS Lab Modules ReviewerDocument4 pagesIS Lab Modules ReviewerMarie MontemarNo ratings yet
- Infinosis™ Presentation - 18062021V1Document20 pagesInfinosis™ Presentation - 18062021V1Meditech visionbdNo ratings yet
- Infinosis PRL IN027705 enDocument2 pagesInfinosis PRL IN027705 enMeditech visionbdNo ratings yet
- Infinosis Troponin I IN047701 enDocument2 pagesInfinosis Troponin I IN047701 enMeditech visionbdNo ratings yet
- Infinosis HCG - IN027702 - enDocument2 pagesInfinosis HCG - IN027702 - enMeditech visionbdNo ratings yet
- Infinosis T3-In017702 enDocument2 pagesInfinosis T3-In017702 enMeditech visionbdNo ratings yet
- Infinosis TSH-In017701 enDocument2 pagesInfinosis TSH-In017701 enMeditech visionbdNo ratings yet
- Infinosis T4-In017703 enDocument2 pagesInfinosis T4-In017703 enMeditech visionbdNo ratings yet
- Infinosis Myo IN047702 enDocument2 pagesInfinosis Myo IN047702 enMeditech visionbdNo ratings yet
- Infinosis HbA1c IN067701 enDocument2 pagesInfinosis HbA1c IN067701 enMeditech visionbdNo ratings yet
- Infinosis IL-6 IN057704 enDocument2 pagesInfinosis IL-6 IN057704 enMeditech visionbdNo ratings yet
- Infinosis FSH IN027704 enDocument2 pagesInfinosis FSH IN027704 enMeditech visionbdNo ratings yet
- Infinosis LH IN027703 enDocument2 pagesInfinosis LH IN027703 enMeditech visionbdNo ratings yet
- Infinosis NT-proBNP IN047705 enDocument2 pagesInfinosis NT-proBNP IN047705 enMeditech visionbdNo ratings yet
- Infinosis PCT IN057701 enDocument2 pagesInfinosis PCT IN057701 enMeditech visionbdNo ratings yet
- Qvî/Qvîxi BVG T Ivj Bs Köbx WelqDocument13 pagesQvî/Qvîxi BVG T Ivj Bs Köbx WelqMeditech visionbdNo ratings yet
- Infinosis MAU IN067703 enDocument2 pagesInfinosis MAU IN067703 enMeditech visionbdNo ratings yet
- Infinosis CK-MB - IN047703 - enDocument2 pagesInfinosis CK-MB - IN047703 - enMeditech visionbdNo ratings yet
- New Microsoft Office Word DocumentDocument3 pagesNew Microsoft Office Word DocumentMeditech visionbdNo ratings yet
- Link 3 TechnologyDocument1 pageLink 3 TechnologyMeditech visionbdNo ratings yet
- Infinosis CRP IN057702 enDocument2 pagesInfinosis CRP IN057702 enMeditech visionbdNo ratings yet
- Welq T KV Ri Avb ': Qvî/Qvîxi BVG T Gvzvi BVG T Wczvi BVG T Ivj Bs TDocument19 pagesWelq T KV Ri Avb ': Qvî/Qvîxi BVG T Gvzvi BVG T Wczvi BVG T Ivj Bs TMeditech visionbdNo ratings yet
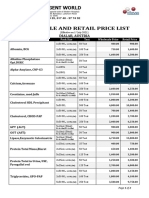
- Price List Wholesale and Retail (June-2021)Document2 pagesPrice List Wholesale and Retail (June-2021)Meditech visionbdNo ratings yet
- 7915 01933902846 101103689110 7be4cszp90Document1 page7915 01933902846 101103689110 7be4cszp90Meditech visionbdNo ratings yet
- Biochemistry Chema, Italy: (IFCC) (IFCC) (IFCC)Document2 pagesBiochemistry Chema, Italy: (IFCC) (IFCC) (IFCC)Meditech visionbdNo ratings yet
- Amylase enDocument1 pageAmylase enRakib Hossain 3A-159No ratings yet
- Price Quotation For Following Lab AccessoriesDocument1 pagePrice Quotation For Following Lab AccessoriesMeditech visionbdNo ratings yet
- Price Quotation For Pathological Reagents. Sir,: (IFCC) (IFCC) (IFCC)Document1 pagePrice Quotation For Pathological Reagents. Sir,: (IFCC) (IFCC) (IFCC)Meditech visionbdNo ratings yet
- Price Quotation For Following Lab AccessoriesDocument1 pagePrice Quotation For Following Lab AccessoriesMeditech visionbdNo ratings yet
- M T Jute Trading: Count & Quality: 10/1 LBS 1 PLY HessianDocument4 pagesM T Jute Trading: Count & Quality: 10/1 LBS 1 PLY HessianMeditech visionbdNo ratings yet
- Toyota's Marketing StrategyDocument14 pagesToyota's Marketing StrategyLavin Gurnani0% (1)
- Black Veil BridesDocument2 pagesBlack Veil BridesElyza MiradonaNo ratings yet
- CENELEC RA STANDARDS CATALOGUEDocument17 pagesCENELEC RA STANDARDS CATALOGUEHamed AhmadnejadNo ratings yet
- Dimension ReductionDocument15 pagesDimension ReductionShreyas VaradkarNo ratings yet
- Sample Id: Sample Id: 6284347 Icmr Specimen Referral Form Icmr Specimen Referral Form For For Covid-19 (Sars-Cov2) Covid-19 (Sars-Cov2)Document2 pagesSample Id: Sample Id: 6284347 Icmr Specimen Referral Form Icmr Specimen Referral Form For For Covid-19 (Sars-Cov2) Covid-19 (Sars-Cov2)Praveen KumarNo ratings yet
- SAC SINGLAS Accreditation Schedule 15 Apr 10Document5 pagesSAC SINGLAS Accreditation Schedule 15 Apr 10clintjtuckerNo ratings yet
- ......... NCP CaseDocument34 pages......... NCP Casevipnikally80295% (19)
- Beef & Dairy 2016Document36 pagesBeef & Dairy 2016The Standard NewspaperNo ratings yet
- Ð.Ð.Á Valvoline Áóìá Áíáâáóç Ñéôóùíáó 9.6.2019: Omaäa ADocument6 pagesÐ.Ð.Á Valvoline Áóìá Áíáâáóç Ñéôóùíáó 9.6.2019: Omaäa AVagelis MoutoupasNo ratings yet
- MPU 2232 Chapter 5-Marketing PlanDocument27 pagesMPU 2232 Chapter 5-Marketing Plandina azmanNo ratings yet
- Fit Friend Business Game StrategiesDocument7 pagesFit Friend Business Game StrategiesSanchit AggarwalNo ratings yet
- Gender Support Plan PDFDocument4 pagesGender Support Plan PDFGender SpectrumNo ratings yet
- Sci10-Q4-M2_104804Document15 pagesSci10-Q4-M2_104804alindongaprilmaeNo ratings yet
- Nad C541iDocument37 pagesNad C541iapi-3837207No ratings yet
- LV 2000L AD2000 11B 16B Metric Dimension Drawing en 9820 9200 06 Ed00Document1 pageLV 2000L AD2000 11B 16B Metric Dimension Drawing en 9820 9200 06 Ed00FloydMG TecnominNo ratings yet
- 41-How To Calculate Air Temp in Unconditioned SpacesDocument3 pages41-How To Calculate Air Temp in Unconditioned Spacesalmig200No ratings yet
- Panasonic SA-HT878Document82 pagesPanasonic SA-HT878immortalwombatNo ratings yet
- Chapter Three 3.0 Research MethodologyDocument5 pagesChapter Three 3.0 Research MethodologyBoyi EnebinelsonNo ratings yet
- 7 Ways of Looking at Grammar China EditDocument20 pages7 Ways of Looking at Grammar China EditAshraf MousaNo ratings yet
- HE HOUSEKEEPING GR11 Q1 MODULE-6-for-teacherDocument25 pagesHE HOUSEKEEPING GR11 Q1 MODULE-6-for-teacherMikaela YtacNo ratings yet
- LEEA-030.2c2 Certificate of Thorough Examination (Multiple Items) (Overseas) (Dev)Document1 pageLEEA-030.2c2 Certificate of Thorough Examination (Multiple Items) (Overseas) (Dev)GaniyuNo ratings yet
- BS 01726-2-2002Document18 pagesBS 01726-2-2002Joana Casta100% (1)
- The History of Coins and Banknotes in Mexico: September 2012Document35 pagesThe History of Coins and Banknotes in Mexico: September 2012Mladen VidovicNo ratings yet
- Epoxy HRDocument5 pagesEpoxy HRMuthuKumarNo ratings yet
- Brake CMMDocument262 pagesBrake CMMvishalsachanameNo ratings yet
- 4-7 The Law of Sines and The Law of Cosines PDFDocument40 pages4-7 The Law of Sines and The Law of Cosines PDFApple Vidal100% (1)
- 1967 2013 PDFDocument70 pages1967 2013 PDFAlberto Dorado Martín100% (1)
- Ebola Research ProposalDocument10 pagesEbola Research ProposalChege AmbroseNo ratings yet
- Problem solving and decision making in nutrition postgraduate studiesDocument55 pagesProblem solving and decision making in nutrition postgraduate studiesteklayNo ratings yet
- Seal Plans As Per API 682Document66 pagesSeal Plans As Per API 682janamuraliNo ratings yet