Professional Documents
Culture Documents
NCIRS Information Sheet - Injection Site Reactions - July 2019
NCIRS Information Sheet - Injection Site Reactions - July 2019
Uploaded by
Residen DV 2022Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
NCIRS Information Sheet - Injection Site Reactions - July 2019
NCIRS Information Sheet - Injection Site Reactions - July 2019
Uploaded by
Residen DV 2022Copyright:
Available Formats
Information
sheet
Injection site reactions
Injection site reactions are the most common adverse events following immunisation.
These include pain, itching, swelling or redness around the site of injection. These
reactions are usually mild and last for 1–2 days.
Rarely, injection site reactions can be quite large and may extend from joint to joint (e.g.
shoulder to elbow) or may cross a joint. These reactions may occur after administration of
any vaccine but are more common after booster doses of diphtheria, tetanus and
pertussis (DTPa/dTpa). The inflammatory changes develop over a few hours following
vaccination, peak at 24 to 48 hours and resolve completely within a week. Decreased
range of limb movement is uncommon, and the individual is systemically well.
Symptomatic relief may include analgesia and cool compress. Moving the limb will
encourage lymphatic drainage and prevent joint stiffness. Avoid putting the arm in a sling.
Large local reactions can be confused with bacterial cellulitis and antibiotics may be
unnecessarily prescribed. Cellulitis post vaccination is extremely uncommon as bacteria
are rarely introduced into tissues, especially with the use of single-dose vials and
single-use injections. Large local reactions do not require antibiotics.
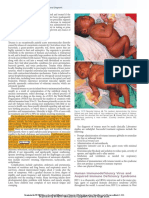
Figure: Injection site reactions
NCIRS Information sheet | Injection site reactions July 2019 1
Large injection site reaction versus cellulitis post vaccination
Large injection site reaction Cellulitis
Tenderness for the first few hours after Tenderness increases as erythema increases.
vaccination which subsides as erythema Once the erythema enlarges to extend joint to
increases in size. joint the limb is exquisitely tender.
Not associated with systemic toxicity. May have Usually accompanied with high-grade or
a mild fever in the first 24 hours which settles. persistent fever, malaise, lethargy
Regional lymphadenopathy may occur and is May be associated with lymphangitis (tracking of
usually non tender. erythema along the lymph vessel) or tender or
non-tender regional lymphadenopathy
Decreased range of limb movement is Decreased range of limb movement is common.
uncommon.
Recommendation for future vaccinations
Booster doses of the same vaccine may cause a similar reaction. There is no increased
risk of anaphylaxis or systemic issues and therefore it is still recommended that children
and adults should continue with the scheduled vaccines for protection against vaccine-
preventable diseases. People should be advised of the potential for a repeat injection site
reaction and how to manage it.
Details on the reporting of adverse events following immunisation and specialist
immunisation clinics in each state and territory can be found at
http://www.ncirs.org.au/health-professionals/specialist-immunisation-services and by
contacting your local public health unit.
For any further advice contact:
Phone: 1800 NSWISS (1800 679 477)
Email: SCHN-NSWISS@health.nsw.gov.au
NCIRS Information sheet | Injection site reactions July 2019 2
You might also like
- Cassey Ho - PIIT 28 3.0 PDFDocument45 pagesCassey Ho - PIIT 28 3.0 PDFWinslow100% (1)
- VancomycinDocument1 pageVancomycinE100% (2)
- Sushrut Samhita Vol IIDocument812 pagesSushrut Samhita Vol IIazteck_indian88% (16)
- Silvadene (Silver Sulfadiazine)Document1 pageSilvadene (Silver Sulfadiazine)ENo ratings yet
- 08 Pathology Orthobullets2017 PDFDocument189 pages08 Pathology Orthobullets2017 PDFdr_shafiq100% (1)
- Nursing 102 Med-Surg PerioperaDocument7 pagesNursing 102 Med-Surg PerioperaKarla FralalaNo ratings yet
- TetanusDocument32 pagesTetanusVinotheran MichaelNo ratings yet
- OET Listening-Official-BookDocument131 pagesOET Listening-Official-BookMonica100% (3)
- Case Study For CellulitisDocument21 pagesCase Study For CellulitisMary Ann Lumbay Paye75% (4)
- The Perfect Neutropenic Diet Cookbook; The Complete Nutrition Guide To Reinstating Overall Health For General Wellness With Delectable And Nourishing RecipesFrom EverandThe Perfect Neutropenic Diet Cookbook; The Complete Nutrition Guide To Reinstating Overall Health For General Wellness With Delectable And Nourishing RecipesNo ratings yet
- Book - Community Medicine MCQSDocument52 pagesBook - Community Medicine MCQSMuhammad Javed Gaba100% (3)
- Obstetrics MCQsDocument12 pagesObstetrics MCQsDeeksha BhardwajNo ratings yet
- The Virus Misconception Part IIDocument56 pagesThe Virus Misconception Part IIFranco Cogniti100% (1)
- Adverse Event Following ImmunizationDocument22 pagesAdverse Event Following ImmunizationLilia Priscilla Tuibuen AureusNo ratings yet
- Childhood Immunization & Catch Up Immunization-2Document53 pagesChildhood Immunization & Catch Up Immunization-2Haters ExterminatorNo ratings yet
- Immunization: Presenter: Ahmad Tahir Siti Nurul Farahah Supervised By: DR MuhaireenDocument40 pagesImmunization: Presenter: Ahmad Tahir Siti Nurul Farahah Supervised By: DR MuhaireenNadia SalwaniNo ratings yet
- Fdar Charting: Date, Time, and Shift Focus Progress NotesDocument4 pagesFdar Charting: Date, Time, and Shift Focus Progress NotesMikaela Gabrielle GERALINo ratings yet
- MeningococcemiaDocument9 pagesMeningococcemiaEunika Castro GarcesNo ratings yet
- LeprosyDocument48 pagesLeprosyPatNo ratings yet
- Identify Common Postoperative Problems and Their ManagementDocument6 pagesIdentify Common Postoperative Problems and Their ManagementSilla, KyshiaNo ratings yet
- Tetanus NeonatorumDocument1 pageTetanus Neonatorumeagame gamersNo ratings yet
- The Immune System: Care of Clients With Altered ImmunityDocument87 pagesThe Immune System: Care of Clients With Altered ImmunityHanni Dayang PuspitasariNo ratings yet
- Covid 19 Vaccine Moderna Epar Product Information enDocument29 pagesCovid 19 Vaccine Moderna Epar Product Information enShreyasri SainNo ratings yet
- Annex I Summary of Product CharacteristicsDocument29 pagesAnnex I Summary of Product CharacteristicslisnerisNo ratings yet
- Adverse Events Following ImmunizationDocument37 pagesAdverse Events Following ImmunizationAkshatha ShivNo ratings yet
- Septic Arthritis & OsteomyelitisDocument42 pagesSeptic Arthritis & OsteomyelitisCabdi WaliNo ratings yet
- Influvac Tetra: New Zealand Data SheetDocument8 pagesInfluvac Tetra: New Zealand Data SheetNeha SaxenaNo ratings yet
- OIT Consent and Info Before VisitDocument6 pagesOIT Consent and Info Before VisitAndres ANo ratings yet
- InfectionDocument8 pagesInfectionMsPocketbook HoarderNo ratings yet
- Abses DMDocument19 pagesAbses DMAngel MakinNo ratings yet
- Viral Skin InfectionsDocument9 pagesViral Skin InfectionsMa Sul JudayaNo ratings yet
- Peran Imunisasi Pada Pasien DewasaDocument30 pagesPeran Imunisasi Pada Pasien DewasararaNo ratings yet
- AEFIDocument24 pagesAEFIadiNo ratings yet
- VITALSIGNSIMMUNIZATIONDocument6 pagesVITALSIGNSIMMUNIZATIONGlaiza Mae TuralbaNo ratings yet
- Contin. Pharma NotesDocument37 pagesContin. Pharma Notesdelie joy dacdacNo ratings yet
- Cellulitis and Erysipelas Antimicrobial Prescribing PDF 66141774778309Document43 pagesCellulitis and Erysipelas Antimicrobial Prescribing PDF 66141774778309Fabián CoitinhoNo ratings yet
- AEFI Training - Presentation Final.1Document56 pagesAEFI Training - Presentation Final.1Minhajul IslamNo ratings yet
- Nurse Practitioner Clinical Protocol: Management of CellulitisDocument11 pagesNurse Practitioner Clinical Protocol: Management of CellulitisJason MarshallNo ratings yet
- ImmuneDocument5 pagesImmuneJameGretNo ratings yet
- COVID-19 After Your VaccineDocument2 pagesCOVID-19 After Your VaccineMatt EbrahimiNo ratings yet
- Moderna Information Sheet-2020-12-29Document2 pagesModerna Information Sheet-2020-12-29TLNo ratings yet
- All Small Letters For Brand Name, First Letter Is Capitalized Bold Dosage Frequency RouteDocument15 pagesAll Small Letters For Brand Name, First Letter Is Capitalized Bold Dosage Frequency RouteNiña Jean Tormis AldabaNo ratings yet
- Effect of Vaccine To PeopleDocument2 pagesEffect of Vaccine To PeopleusernamerainNo ratings yet
- Erythromycin & Pneumococcal VaccineDocument6 pagesErythromycin & Pneumococcal VaccineNikki Joy NavarroNo ratings yet
- Pott'S Disease: DescriptionDocument8 pagesPott'S Disease: DescriptiongoyaNo ratings yet
- Adult 1 Test 1 Study GuideDocument9 pagesAdult 1 Test 1 Study GuideChristopher JamesNo ratings yet
- Herpes ZosterDocument3 pagesHerpes ZosterKathleen Marie ChuangNo ratings yet
- Group 3 - ImmunizationDocument74 pagesGroup 3 - ImmunizationNathanieGequilloNo ratings yet
- DRUGDocument22 pagesDRUGAbel MncaNo ratings yet
- Antibiotics Friend or FoeDocument3 pagesAntibiotics Friend or FoeInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Brand Name: Berirab Contents: Indication / Action:: RabiesDocument4 pagesBrand Name: Berirab Contents: Indication / Action:: RabiesAbdelmar SusulanNo ratings yet
- Acute Infections Oral and Paraoral TissuesDocument33 pagesAcute Infections Oral and Paraoral TissuesKarthik S'twinkles SNo ratings yet
- Study File ImportantDocument12 pagesStudy File Importantsami khanNo ratings yet
- Infectious DermatosesDocument41 pagesInfectious DermatosesJuma SammyNo ratings yet
- Awareness On Chikungunya.Document53 pagesAwareness On Chikungunya.Mizanur RahmanNo ratings yet
- AllergensDocument3 pagesAllergensDE LEON ALLIANA MARIENo ratings yet
- Case 3Document27 pagesCase 3Eduard GarchitorenaNo ratings yet
- Case Study 2Document7 pagesCase Study 2desdav100% (1)
- OsteomyelitisDocument7 pagesOsteomyelitis4kscribd100% (1)
- 9) Medical Complications of Drug TakingDocument44 pages9) Medical Complications of Drug TakingDr. Zirwa AsimNo ratings yet
- Annex I Summary of Product CharacteristicsDocument32 pagesAnnex I Summary of Product Characteristicscalin_tudor_1No ratings yet
- Uveitis - Treatment - UpToDateDocument12 pagesUveitis - Treatment - UpToDateRohit RajeevanNo ratings yet
- IDflu LeafletDocument28 pagesIDflu LeafletEllaNo ratings yet
- Poorly Nourished Elderly Obese Impaired Immune System Chronic Illness Receiving Long Term Corticosteroid TherapyDocument182 pagesPoorly Nourished Elderly Obese Impaired Immune System Chronic Illness Receiving Long Term Corticosteroid TherapyjhaninahNo ratings yet
- Reumathoid ArthritisDocument11 pagesReumathoid ArthritisYAny R Setyawati100% (1)
- Chapter 10Document32 pagesChapter 10Henderi SaputraNo ratings yet
- 5-Fluorouracil, 5-FU Efudex Antineoplastic or CytotoxicDocument5 pages5-Fluorouracil, 5-FU Efudex Antineoplastic or CytotoxicEzra TuanNo ratings yet
- Case Study BurnDocument3 pagesCase Study BurnInday BebeNo ratings yet
- Toxic Epidermal Necrolysis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandToxic Epidermal Necrolysis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Fear of Being AloneDocument28 pagesFear of Being AloneHà Anh Lê VũNo ratings yet
- Novel Uses of Skin Biopsy Punches in DermatosurgeryDocument6 pagesNovel Uses of Skin Biopsy Punches in DermatosurgerynoviNo ratings yet
- 2017 Guidance For The Conduct of JBI Scoping Reviews: September 2017Document43 pages2017 Guidance For The Conduct of JBI Scoping Reviews: September 2017Biruk BayeNo ratings yet
- The Role of Probability - BostonDocument39 pagesThe Role of Probability - BostonThai CheNo ratings yet
- Skenario Baru 4Document6 pagesSkenario Baru 4Nabila SalsabilaNo ratings yet
- Merenstein Gardners Handbook of Neonatal Intensive Care 8Th Edition Sandra Lee Gardner Brian S Carter Mary I Enzman Hines Jacinto A Hernandez Download PDF ChapterDocument52 pagesMerenstein Gardners Handbook of Neonatal Intensive Care 8Th Edition Sandra Lee Gardner Brian S Carter Mary I Enzman Hines Jacinto A Hernandez Download PDF Chapterrichard.martin380100% (17)
- The Ai Revolution in Medicine GPT 4 and Beyond Peter Lee 3 Full ChapterDocument67 pagesThe Ai Revolution in Medicine GPT 4 and Beyond Peter Lee 3 Full Chapterrobert.lee850100% (8)
- Drugs Acting On Digestive System of AnimalsDocument32 pagesDrugs Acting On Digestive System of AnimalsSunil100% (3)
- A Simple and Fast Experimental Model: Laboratory InvestigationDocument4 pagesA Simple and Fast Experimental Model: Laboratory InvestigationAjay PeddiNo ratings yet
- 6 IJBSM April 2021 Maneesha Et Al-4Document1 page6 IJBSM April 2021 Maneesha Et Al-4vc_mpNo ratings yet
- Clinical Skills 2018 Big Guide PDFDocument100 pagesClinical Skills 2018 Big Guide PDFDaniel TanNo ratings yet
- Quick Facts - Debridement and Chronic Wounds 1Document2 pagesQuick Facts - Debridement and Chronic Wounds 1FadliNo ratings yet
- 100 Pages of MCQs LJDocument68 pages100 Pages of MCQs LJPeter BoatengNo ratings yet
- Pre-Hospital Trauma Life Support Date: Name: I.D. Number: Activity 2 Head and Face Injuries Motor Vehicle AccidentDocument3 pagesPre-Hospital Trauma Life Support Date: Name: I.D. Number: Activity 2 Head and Face Injuries Motor Vehicle Accidentamal abdulrahmanNo ratings yet
- SHT Acute Respiratory Infections (CHN)Document10 pagesSHT Acute Respiratory Infections (CHN)Chrissan Pio LaderaNo ratings yet
- Unexplained Gastrointestinal Symptoms After Abuse in A Prospective333 Study of Children at Risk For Abuse and NeglectDocument7 pagesUnexplained Gastrointestinal Symptoms After Abuse in A Prospective333 Study of Children at Risk For Abuse and NeglectAyy LomaNo ratings yet
- EHE PPT (OHS)Document29 pagesEHE PPT (OHS)Ramzi JamalNo ratings yet
- The Guidelines: A Framework For Safe Drinking-WaterDocument15 pagesThe Guidelines: A Framework For Safe Drinking-WaterAMMARNo ratings yet
- !DRUGSTUDY CasepresssDocument6 pages!DRUGSTUDY CasepresssAchi AxxxNo ratings yet
- The Effects of Lifestyle For The Management of Patient With Diabetes Mellitus Type 2Document33 pagesThe Effects of Lifestyle For The Management of Patient With Diabetes Mellitus Type 2EduNo ratings yet
- Dincharya - Ashtang HridayaDocument5 pagesDincharya - Ashtang HridayaDhanwantari JhaNo ratings yet