Professional Documents
Culture Documents
5.12: Chemical Shifts in / ( 1H/) NMR Spectroscopy: Hydrogen Type Chemical Shift (PPM)
Uploaded by
Lara DiasOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
5.12: Chemical Shifts in / ( 1H/) NMR Spectroscopy: Hydrogen Type Chemical Shift (PPM)
Uploaded by
Lara DiasCopyright:
Available Formats
5.
12: Chemical Shifts in \(^1H\) NMR Spectroscopy
Objectives
After completing this section, you should be able to
1. state the approximate chemical shift (δ) for the following types of protons:
a. aromatic.
b. vinylic.
c. those bonded to carbon atoms which are in turn bonded to a highly electronegative element.
d. those bonded to carbons which are next to unsaturated centres.
e. those bonded to carbons which are part of a saturated system.
2. predict the approximate chemical shifts of each of the protons in an organic compound, given its structure and a
table of chemical shift correlations.
Study Notes
You should not attempt to memorize the chemical shifts listed in the table of this section, although it is probable that
you will need to refer to it quite frequently throughout the remainder of this course. To fulfil Objective 1, above, you
should be familiar with the information presented in the figure of chemical shift ranges for organic compounds. If you
have an approximate idea of the chemical shifts of some of the most common types of protons, you will find the
interpretation of 1H NMR spectra less arduous than it might otherwise be. Notice that we shall not try to understand
why aromatic protons are deshielded or why alkynyl protons are not deshielded as much as vinylic protons. These
phenonomena can be explained, but the focus is on the interpretation of 1H NMR spectra, not on the underlying theory.
1
H NMR Chemical Shifts
Chemical shift is associated with the Larmor frequency of a nuclear spin to its chemical environment.
Tetramethylsilan[TMS;(CH3)4Si] is generally used for standard to determine chemical shift of compounds: δTMS=0ppm. In
other words, frequencies for chemicals are measured for a 1H or 13C nucleus of a sample from the 1H or 13C resonance of
TMS. It is important to understand trend of chemical shift in terms of NMR interpretation. The proton NMR chemical shift
is affect by nearness to electronegative atoms (O, N, halogen.) and unsaturated groups (C=C,C=O, aromatic).
Electronegative groups move to the down field (left; increase in ppm). Unsaturated groups shift to downfield (left) when
affecting nucleus is in the plane of the unsaturation, but reverse shift takes place in the regions above and below this plane.
1H chemical shift play a role in identifying many functional groups. Figure 1. indicates important example to figure out the
functional groups.
Figure 1. 1H chemical shift ranges for organic compounds
Chemical shift values are in parts per million (ppm) relative to tetramethylsilane.
Hydrogen type Chemical shift (ppm)
5.12.1 1/21/2022 https://chem.libretexts.org/@go/page/167098
RCH3 0.9 - 1.0
RCH2R 1.2 - 1.7
R3CH 1.5 – 2.0
2.0 – 2.3
1.5 – 1.8
RNH2 1-3
ArCH3 2.2 – 2.4
2.3 – 3.0
ROCH3 3.7 – 3.9
3.7 – 3.9
ROH 1-5
3.7 – 6.5
5-9
ArH 6.0 – 8.7
9.5 – 10.0
10 - 13
Exercise
Questions
Q13.9.1
The following have one H1 NMR peak. In each case predict approximately where this peak would be in a spectra.
5.12.2 1/21/2022 https://chem.libretexts.org/@go/page/167098
Q13.9.2
Identify the different equivalent protons in the following molecule and predict their expected chemical shift.
Solutions
S13.9.1
A. 5.20 δ; B. 1.50 δ; C. 6.40 δ; D. 1.00 δ
S13.9.2
There are 6 different protons in this molecule
The shifts are (close) to the following: (a) 2 δ; (b) 6 δ; (c) 6.5 δ; (d) 7 δ; (e) 7.5 δ; (f) 7 δ
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
5.12.3 1/21/2022 https://chem.libretexts.org/@go/page/167098
You might also like
- Organic Spectroscopic AnalysisDocument186 pagesOrganic Spectroscopic AnalysisDorin Popescu100% (1)
- Astronomy FormulaeDocument36 pagesAstronomy Formulaeescherer1099100% (3)
- 2d NMRDocument32 pages2d NMRDelicz TanNo ratings yet
- ch09 TestbankDocument84 pagesch09 TestbankPatrick Malcolm Santos80% (5)
- Plasma AntennaDocument21 pagesPlasma AntennaAnush Pagadala100% (1)
- ch9 Test-BankDocument85 pagesch9 Test-Bank박훈희No ratings yet
- NMR HandoutDocument23 pagesNMR HandoutVirendra Singh RajputNo ratings yet
- NMR Spectroscopy - Short NoteDocument6 pagesNMR Spectroscopy - Short Notecoolhemakumar100% (4)
- 16.1 Multiple-Choice Questions: Chapter 16 Carboxylic Acids and EstersDocument23 pages16.1 Multiple-Choice Questions: Chapter 16 Carboxylic Acids and EstersJames ChavezNo ratings yet
- Schultz 1962Document14 pagesSchultz 1962Diego1980b100% (1)
- PMR Spectroscopy: Solved Problems Volume : IIFrom EverandPMR Spectroscopy: Solved Problems Volume : IIRating: 5 out of 5 stars5/5 (3)
- Structure Determination of Organic Compounds - ChemvoiceDocument37 pagesStructure Determination of Organic Compounds - ChemvoiceJubin Kumar0% (1)
- Introduction To Proton NMR SpectroscopyNJTDocument13 pagesIntroduction To Proton NMR SpectroscopyNJTNicoleNo ratings yet
- Chemical ShiftDocument25 pagesChemical ShiftArchana VanjariNo ratings yet
- Chapter 14Document8 pagesChapter 14nelaojNo ratings yet
- MagResonChem 1999 37 393Document8 pagesMagResonChem 1999 37 393juanvi.sanchoNo ratings yet
- Simulation - ": Gas-Phase Inorganic Chemlstry: Laser Spectroscopy of Calcium and Strontium Monopyrrolate MoleculesDocument4 pagesSimulation - ": Gas-Phase Inorganic Chemlstry: Laser Spectroscopy of Calcium and Strontium Monopyrrolate MoleculesDamxz5No ratings yet
- CHE323 FS18 Teil1 - PDF PDFDocument114 pagesCHE323 FS18 Teil1 - PDF PDFreauhanNo ratings yet
- Lecture 1Document28 pagesLecture 1Sebastián Castro FragueiroNo ratings yet
- 2D NMR SpectrosDocument77 pages2D NMR SpectrosRitul VasaniNo ratings yet
- Analytical TechniquesDocument6 pagesAnalytical TechniquesahumanbeinginearthNo ratings yet
- T186266 - AvanceCore NMR Spectra in 15 Minutes Application Note - FINALDocument4 pagesT186266 - AvanceCore NMR Spectra in 15 Minutes Application Note - FINALLaiza Bruzadelle LoureiroNo ratings yet
- Solvent-Dependent Structure of Iridium Dihydride Complexes Different Geometries at Low and High Dielectricity of The MediumDocument10 pagesSolvent-Dependent Structure of Iridium Dihydride Complexes Different Geometries at Low and High Dielectricity of The Mediumxuyijing2007comNo ratings yet
- NMR InterpretationDocument8 pagesNMR InterpretationSandip FirkeNo ratings yet
- Model Answer: The Following Questions Answer Choose The Correct Answer: (20Document4 pagesModel Answer: The Following Questions Answer Choose The Correct Answer: (20Khalid AbeedNo ratings yet
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Overview of Applications in Chemical BiologyDocument24 pagesNuclear Magnetic Resonance (NMR) Spectroscopy: Overview of Applications in Chemical BiologyazzaassNo ratings yet
- DT261/3, DT299/3: Dr. Andrew KnoxDocument45 pagesDT261/3, DT299/3: Dr. Andrew KnoxkeatyNo ratings yet
- New Frontiers in Sciences, Engineering and the Arts: Vol. Ii the Chemistry of Initiation of Non-Ringed Monomers/CompoundsFrom EverandNew Frontiers in Sciences, Engineering and the Arts: Vol. Ii the Chemistry of Initiation of Non-Ringed Monomers/CompoundsNo ratings yet
- NMRDocument16 pagesNMRChandni SahaNo ratings yet
- Structure Determination: Nuclear Magnetic Resonance SpectrosDocument33 pagesStructure Determination: Nuclear Magnetic Resonance Spectros張湧浩No ratings yet
- Chem 201 - Paper B - Nov 2021Document7 pagesChem 201 - Paper B - Nov 2021arnomasvosveNo ratings yet
- 10 1021@ja0121540Document9 pages10 1021@ja0121540Hamza MalikNo ratings yet
- NMR 7.Document36 pagesNMR 7.Imran KhanNo ratings yet
- Chemistry I (CH 101orch201)Document5 pagesChemistry I (CH 101orch201)Singham shekNo ratings yet
- NMR Spectroscopy LabDocument7 pagesNMR Spectroscopy LabBriana Halbert100% (2)
- Radical Stability-Thermochemical Aspects: Johnny Hioe and Hendrik ZipseDocument27 pagesRadical Stability-Thermochemical Aspects: Johnny Hioe and Hendrik ZipseNaman GuptaNo ratings yet
- H-1 NMR: Low Resolution: Chemical ShiftsDocument1 pageH-1 NMR: Low Resolution: Chemical ShiftsKhondokar TarakkyNo ratings yet
- NMR Kinetics: Study of A Reversible Hydrolysis ReactionDocument8 pagesNMR Kinetics: Study of A Reversible Hydrolysis ReactionOldbooklover100% (2)
- Wolf and LambDocument10 pagesWolf and LambPrasanna IyerNo ratings yet
- Role of Solvent Reorganization Dynamics in Electron-Transfer Processes. Anomalous Kinetic Behavior in Alcohol SolventsDocument8 pagesRole of Solvent Reorganization Dynamics in Electron-Transfer Processes. Anomalous Kinetic Behavior in Alcohol Solventsenaveen2005No ratings yet
- Solvent Exchange On Metal Ions: Advances in Inorganic Chemistry VOLUME 54 ISSN 0898 - 8838 All Rights ReservedDocument69 pagesSolvent Exchange On Metal Ions: Advances in Inorganic Chemistry VOLUME 54 ISSN 0898 - 8838 All Rights Reservedgeo angNo ratings yet
- 1 s2.0 S0584854721001749 Main PDFDocument6 pages1 s2.0 S0584854721001749 Main PDFxxxNo ratings yet
- Assignment NMR-spectrosDocument4 pagesAssignment NMR-spectrosShovan SwainNo ratings yet
- Question Bank of Chemistry (BSC-105) For 2018 Onwards Batch StudentsDocument8 pagesQuestion Bank of Chemistry (BSC-105) For 2018 Onwards Batch Studentsinterestingfacts2525No ratings yet
- Modelling 1H NMR Spectra of Organic Compounds: Theory, Applications and NMR Prediction SoftwareFrom EverandModelling 1H NMR Spectra of Organic Compounds: Theory, Applications and NMR Prediction SoftwareNo ratings yet
- BT6604 Cre PDFDocument56 pagesBT6604 Cre PDFNIKHIL SHINDENo ratings yet
- Spectroscopy Problems-MaleczkaDocument33 pagesSpectroscopy Problems-MaleczkaBruna SofiaNo ratings yet
- RH Catalizador RAGNDocument19 pagesRH Catalizador RAGNCAMILO SEBASTIAN CATACORA REVOLLONo ratings yet
- Chapter 5Document65 pagesChapter 5kenethlumu9No ratings yet
- Chemical KineticsDocument69 pagesChemical KineticsDaniel YakubovichNo ratings yet
- Q NmrH1highresDocument5 pagesQ NmrH1highresKhondokar TarakkyNo ratings yet
- 2004-Slow Dynamics of A Confined Supercooled Binary Mixture in Comparison With The Bulk PhaseDocument9 pages2004-Slow Dynamics of A Confined Supercooled Binary Mixture in Comparison With The Bulk Phasepreethim6462No ratings yet
- Conformational Analysis of Methyl P-Cellobioside by ROESY NMR Spectroscopy and MD Simulations in Combination With The CROSREL MethodDocument22 pagesConformational Analysis of Methyl P-Cellobioside by ROESY NMR Spectroscopy and MD Simulations in Combination With The CROSREL MethodNabiila RahmaniNo ratings yet
- New Terpendole Congeners, Inhibitors of Sterol O-Acyltransferase, Produced by Volutella Citrinella BF - 0440Document20 pagesNew Terpendole Congeners, Inhibitors of Sterol O-Acyltransferase, Produced by Volutella Citrinella BF - 0440Ruba RadNo ratings yet
- SAT Practice 3Document12 pagesSAT Practice 3Lebron JamesNo ratings yet
- Journal of Physics: Conference Series: What Is The Structure of Liquid Bismuth?Document11 pagesJournal of Physics: Conference Series: What Is The Structure of Liquid Bismuth?Guy MakovNo ratings yet
- Applications of NMR Spectroscopy in Petroleum Chemistry: January 2010Document47 pagesApplications of NMR Spectroscopy in Petroleum Chemistry: January 2010Archana JoshiNo ratings yet
- Unknowns Workshop Booklet 2014Document34 pagesUnknowns Workshop Booklet 2014Tales FernandoNo ratings yet
- Topic 20 NotesDocument21 pagesTopic 20 NotesAmmaarah PatelNo ratings yet
- Dissertation NMRDocument5 pagesDissertation NMRFindSomeoneToWriteMyCollegePaperUK100% (1)
- Chemical Kinetic Data Base For Combustion Chemistry. Part I. Methane and ReDocument192 pagesChemical Kinetic Data Base For Combustion Chemistry. Part I. Methane and ReMuhammad AtharNo ratings yet
- Simulation Interactions Diagram Report: Jobname Entry TitleDocument10 pagesSimulation Interactions Diagram Report: Jobname Entry TitlepaiaravindNo ratings yet
- Hydrolysis of Oil of Wintergreen1Document16 pagesHydrolysis of Oil of Wintergreen1uthu_megaNo ratings yet
- The Chemistry of Membranes Used in Fuel Cells: Degradation and StabilizationFrom EverandThe Chemistry of Membranes Used in Fuel Cells: Degradation and StabilizationShulamith SchlickNo ratings yet
- NMR SolventsDocument2 pagesNMR SolventsAnonymous Bh6y0TuVHiNo ratings yet
- 4.8: Acid-Base Extraction: How They WorkDocument5 pages4.8: Acid-Base Extraction: How They WorkLara DiasNo ratings yet
- NMR - Interpretation: Strategy For Solving StructureDocument8 pagesNMR - Interpretation: Strategy For Solving StructureLara DiasNo ratings yet
- TLC Staining Procedure TLC Stain Recipe Stain Chemistry / Physics CommentsDocument3 pagesTLC Staining Procedure TLC Stain Recipe Stain Chemistry / Physics CommentsLara DiasNo ratings yet
- NMRshifts1H GeneralDocument1 pageNMRshifts1H GeneralJeric CestinaNo ratings yet
- Experiment 6Document6 pagesExperiment 6Mama ChoiiNo ratings yet
- Non Drag ForcesDocument5 pagesNon Drag ForcesRelining MineralsNo ratings yet
- Heat Transfer: Mechanical EngineeringDocument10 pagesHeat Transfer: Mechanical EngineeringVenkatasairamreddy KandulaNo ratings yet
- Synthesis and Characterization of Nano Banana Fibre Reinforced Polymer Nano CompositesDocument133 pagesSynthesis and Characterization of Nano Banana Fibre Reinforced Polymer Nano CompositesBoopathi RajaNo ratings yet
- Proceeding Book BioTechBioChem 2020Document8 pagesProceeding Book BioTechBioChem 2020uvir iitmNo ratings yet
- 2005 June Physics 2h PDFDocument24 pages2005 June Physics 2h PDFTatenda ChimwandaNo ratings yet
- Product and Company Identification: Safety Data SheetDocument8 pagesProduct and Company Identification: Safety Data SheetAhmed KhameisNo ratings yet
- Gas Laws (Notes) PDFDocument9 pagesGas Laws (Notes) PDFHassan Jamal100% (1)
- Blueshield™ La T-91 C60 Ni1: Low-Alloy SteelDocument1 pageBlueshield™ La T-91 C60 Ni1: Low-Alloy SteelSimNo ratings yet
- Hydraulic Components and Systems (2012)Document203 pagesHydraulic Components and Systems (2012)bach.leconmeomapNo ratings yet
- Cerium ComplexesDocument16 pagesCerium Complexessch203No ratings yet
- 9science 9 Force and Laws of MotionDocument27 pages9science 9 Force and Laws of MotionMohammed AadilNo ratings yet
- CH 20-21 Answers (All)Document36 pagesCH 20-21 Answers (All)Thục NghiNo ratings yet
- KU SPH 101 Electricity and Magnetism 1 NotesDocument107 pagesKU SPH 101 Electricity and Magnetism 1 NotesCallum Fallen100% (1)
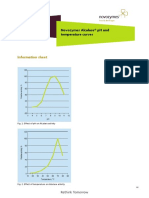
- Alcalase 2.5 L - PH and TemperatureDocument2 pagesAlcalase 2.5 L - PH and TemperatureAída MorenoNo ratings yet
- Niper Jee SyllabusDocument6 pagesNiper Jee Syllabuskumar HarshNo ratings yet
- Thermodynamics 2018 (Repaired)Document15 pagesThermodynamics 2018 (Repaired)carolNo ratings yet
- Aits 2021 FT Ix Jeem.Document16 pagesAits 2021 FT Ix Jeem.Atharv AtoleNo ratings yet
- Steam Generation - Distribution 2023Document64 pagesSteam Generation - Distribution 2023Aaqil cassimNo ratings yet
- ACID BAse AssignmentDocument11 pagesACID BAse AssignmentMosfiqur Rahman100% (2)
- Biochemistry: Molecular and Cell BiochemistryDocument7 pagesBiochemistry: Molecular and Cell BiochemistryKHIENT CARLO MARTINNo ratings yet
- ChemistryDocument4 pagesChemistryMalik Ameer Hamza BalochNo ratings yet
- Cavitation ErosionDocument7 pagesCavitation Erosion82ghost82No ratings yet
- GO 4 Preparation of Carboxylic AcidDocument18 pagesGO 4 Preparation of Carboxylic AcidcikaifaNo ratings yet
- Lecture 23: Introduction To Valence Bond TheoryDocument18 pagesLecture 23: Introduction To Valence Bond TheoryElectro_LiteNo ratings yet