Professional Documents
Culture Documents
NEW - Konvalesen Plasma - PRISMA 2009 Flow Diagram
Uploaded by
Reynardi SutantoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
NEW - Konvalesen Plasma - PRISMA 2009 Flow Diagram
Uploaded by
Reynardi SutantoCopyright:
Available Formats
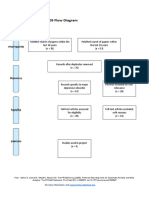
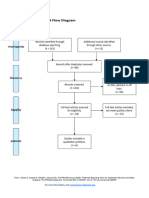
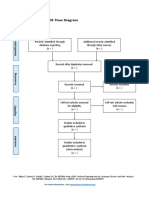
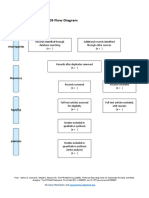
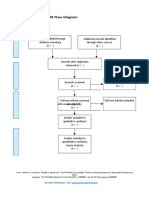
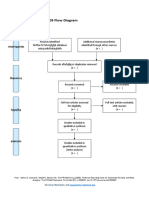
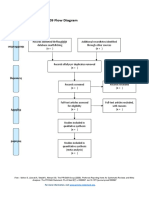
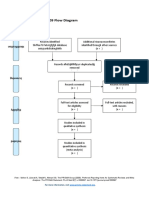
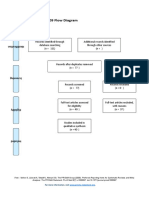
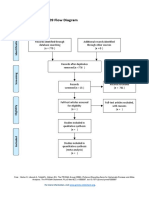
PRISMA 2009 Flow Diagram
Pubmed Embase
Identification (n = 44) (n = 54)
Records after duplicates removed
(n = 61)
Screening
Titles and abstracts Records excluded
screened (n = 36)
(n = 61)
Full-text articles assessed Full-text articles excluded
for eligibility (n = 20)
Eligibility (n = 25) 5 studies with
inappropriate designs
(2 narrative reviews, 3
commentaries/
Studies assessed for data opinion pieces)
dependence (n = 5) 13 studies with
5 observational studies inappropriate settings
(not suitable for home
use during pandemic)
Included
2 irretrievable full-text
Studies included (n = 5) articles (i.e.
5 observational studies conference abstracts)
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-
Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
For more information, visit www.prisma-statement.org.
You might also like
- Techniques in Wildlife Investigations: Design and Analysis of Capture DataFrom EverandTechniques in Wildlife Investigations: Design and Analysis of Capture DataRating: 5 out of 5 stars5/5 (1)
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitabdulNo ratings yet
- PRISMA 2009 Flow Diagram PreenchidoDocument1 pagePRISMA 2009 Flow Diagram PreenchidoSDNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, Visitfadilatul munawwarohNo ratings yet
- Additional Records IdentifiedDocument1 pageAdditional Records IdentifiedSebastian VanegasNo ratings yet
- PRISMA 2009 Flow Diagram IdentificationDocument1 pagePRISMA 2009 Flow Diagram IdentificationAndrés Felipe Obregón HernándezNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitManJ_mchNo ratings yet
- PRISMA Flow DiagramDocument1 pagePRISMA Flow DiagramavfNo ratings yet
- PRISMA 2009 Flow Diagram MS WordDocument1 pagePRISMA 2009 Flow Diagram MS WordYuliza ArianiNo ratings yet
- PRISMA 2009 Flow Diagram PreenchidoDocument1 pagePRISMA 2009 Flow Diagram PreenchidoSDNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepseNo ratings yet
- PRISMA 2009 Flow DiagramDocument1 pagePRISMA 2009 Flow DiagramAndrés Felipe Obregón HernándezNo ratings yet
- PRISMA 2009 Flow Diagram GuideDocument1 pagePRISMA 2009 Flow Diagram GuideKou YashiroNo ratings yet
- Bmjopen 2020 December 10 12 Inline Supplementary Material 3Document1 pageBmjopen 2020 December 10 12 Inline Supplementary Material 3Alessandra Miranda PadillaNo ratings yet
- PRISMA 2009 Flow DiagramDocument1 pagePRISMA 2009 Flow DiagramnurulNo ratings yet
- PRISMA Diagram TemplateDocument1 pagePRISMA Diagram TemplateRitashree DasguptaNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegututsgsgsNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegsgsgsNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegututsgsgskhkhNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegsgsgsNo ratings yet
- PRISMA 2009 Flow Diagram Guide for Systematic Literature ReviewsDocument1 pagePRISMA 2009 Flow Diagram Guide for Systematic Literature ReviewspedriyepseNo ratings yet
- ROSES Flow Diagram For Systematic ReviewsDocument1 pageROSES Flow Diagram For Systematic Reviewswhy daNo ratings yet
- PRISMA 2009 Flow DiagramDocument1 pagePRISMA 2009 Flow DiagrampedriyepsegsgsgsNo ratings yet
- PRISMA 2009 Flow Diagram PDFDocument1 pagePRISMA 2009 Flow Diagram PDFKalis WarenNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegututsgsgskhkhNo ratings yet
- PRISMA 2020 Flow Diagram For Updated Systematic Reviews Which Included Searches of Databases, Registers and Other SourcesDocument1 pagePRISMA 2020 Flow Diagram For Updated Systematic Reviews Which Included Searches of Databases, Registers and Other SourcesSurya WijayaNo ratings yet
- PRISMA Rev3Document1 pagePRISMA Rev3Gosia StarzynskaNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegututsgsgskhkhNo ratings yet
- PRISMA 2009 Flow Diagram GuideDocument1 pagePRISMA 2009 Flow Diagram GuidepedriyepsegututsgsgskhkhNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitRahma Aulia KhairunnisaNo ratings yet
- Prisma PediculosisDocument1 pagePrisma PediculosisAni TikaNo ratings yet
- Flow ChartDocument2 pagesFlow Chartgeorgiana tangkilisanNo ratings yet
- PRISMA 2009 Flow Diagram: 1. Youth, or Adolescents (N 100) 2. Irrelevant With Tubercolusi (N 35) 3Document1 pagePRISMA 2009 Flow Diagram: 1. Youth, or Adolescents (N 100) 2. Irrelevant With Tubercolusi (N 35) 3aisah andiniNo ratings yet
- Bagan Abstrak IACDocument1 pageBagan Abstrak IACJessica OtniellaNo ratings yet
- Prisma PediculosisDocument1 pagePrisma PediculosisAni TikaNo ratings yet
- Clinical Effectiveness of Chin Cup Treatment For TDocument14 pagesClinical Effectiveness of Chin Cup Treatment For TIlzze Zuñigha100% (1)
- PRISMA - 2020 - Flow - Diagram - Updated. JK Menambah Yg SDH Ada. TP Register OnlyDocument1 pagePRISMA - 2020 - Flow - Diagram - Updated. JK Menambah Yg SDH Ada. TP Register Onlyfachry albabNo ratings yet
- PRISMA 2009 Flow DiagramDocument1 pagePRISMA 2009 Flow DiagramsamiratumananNo ratings yet
- PRISMA Flow Diagram Self Care Chronic Kidney DiseaseDocument1 pagePRISMA Flow Diagram Self Care Chronic Kidney DiseaseimranNo ratings yet
- PRISMADocument1 pagePRISMAGosia StarzynskaNo ratings yet
- PRISMA 2009 Flow DiagramDocument1 pagePRISMA 2009 Flow Diagrammoutasim mohammadNo ratings yet
- PRISMA 2020 Flow Diagram New SRs v2Document1 pagePRISMA 2020 Flow Diagram New SRs v2Ivan PradhanaNo ratings yet
- ROSES Flow Diagram For Systematic MapsDocument1 pageROSES Flow Diagram For Systematic MapsLina DiazNo ratings yet
- GROUP 5 PRISMA (Final)Document2 pagesGROUP 5 PRISMA (Final)Queen RosieNo ratings yet
- ROSES Flow Diagram For Systematic MapsDocument1 pageROSES Flow Diagram For Systematic MapsJoanne WongNo ratings yet
- PRISMA 2009 Flow Diagram PDFDocument1 pagePRISMA 2009 Flow Diagram PDFসোমনাথ মহাপাত্রNo ratings yet
- Unit X - Final Review - 1 Per PageDocument30 pagesUnit X - Final Review - 1 Per PageKase1No ratings yet
- Systematic literature search resultsDocument1 pageSystematic literature search resultsniar196No ratings yet
- PRISMA 2009 Flow Diagram MS WordDocument1 pagePRISMA 2009 Flow Diagram MS Wordfelicia081585343669No ratings yet
- PRISMA 2020 Flow Diagram New SRs v1Document1 pagePRISMA 2020 Flow Diagram New SRs v1Krisna ayuNo ratings yet
- T 3Document1 pageT 3Cynthia Collao TeránNo ratings yet
- Tugas Ebp FixDocument18 pagesTugas Ebp FiximranNo ratings yet
- Guidelines for managing acute respiratory distress syndromeDocument20 pagesGuidelines for managing acute respiratory distress syndromeMica MariaNo ratings yet
- PRISMA - Urban e KhouryDocument1 pagePRISMA - Urban e KhourypasqualiniericaNo ratings yet
- Statistical TestDocument4 pagesStatistical TestHà ChiNo ratings yet
- PRISMA 2020 Flow Diagram For New Systematic Reviews Which Included Searches of Databases, Registers and Other SourcesDocument1 pagePRISMA 2020 Flow Diagram For New Systematic Reviews Which Included Searches of Databases, Registers and Other SourcesSurya WijayaNo ratings yet
- MR Steve Turner, DR Steve Macgillivray, Ms Sheela TripatheeDocument1 pageMR Steve Turner, DR Steve Macgillivray, Ms Sheela TripatheemebibegNo ratings yet
- 1 - Design of Experiments (29-12-2023)Document34 pages1 - Design of Experiments (29-12-2023)zinaNo ratings yet
- Channel CapDocument9 pagesChannel CapDeepika RastogiNo ratings yet
- +1 TM Slow Learner Material For Reduced Portion 2021-22Document55 pages+1 TM Slow Learner Material For Reduced Portion 2021-22Prasanth Prasanth100% (2)
- FoCal Multi-class Toolkit GuideDocument32 pagesFoCal Multi-class Toolkit Guidethyagosmesme100% (1)
- IELTS PART 1 (Autoguardado)Document8 pagesIELTS PART 1 (Autoguardado)CARLOS CAICEDONo ratings yet
- FINA2209 Financial Planning: Week 3: Indirect Investment and Performance MeasurementDocument43 pagesFINA2209 Financial Planning: Week 3: Indirect Investment and Performance MeasurementDylan AdrianNo ratings yet
- Fullpapers Kmp31ce4c51eafullDocument11 pagesFullpapers Kmp31ce4c51eafullyohana biamnasiNo ratings yet
- Media Palnning ProcessDocument3 pagesMedia Palnning ProcessSrinivas KumarNo ratings yet
- Magnetic Particle TestDocument4 pagesMagnetic Particle TestHarry Doble100% (1)
- 20NCT2 1784 SampleDocument12 pages20NCT2 1784 Samplekimjohn dejesusNo ratings yet
- "Hybrid" Light Steel Panel and Modular Systems PDFDocument11 pages"Hybrid" Light Steel Panel and Modular Systems PDFTito MuñozNo ratings yet
- Colloquium: A Learner-Centric View of Mobile Seamless Learning Lung-Hsiang WongDocument5 pagesColloquium: A Learner-Centric View of Mobile Seamless Learning Lung-Hsiang WongWayne LeungNo ratings yet
- BABOK V3 10. Glossary PDFDocument16 pagesBABOK V3 10. Glossary PDFAakash ChhibberNo ratings yet
- Licence Acoknowledgement SlipDocument1 pageLicence Acoknowledgement SlipBicky ChoudhuryNo ratings yet
- IRS Form 13909Document2 pagesIRS Form 13909whoiscolleenlynnNo ratings yet
- How To Combine Cells Into A Cell With Comma, Space and Vice VersaDocument8 pagesHow To Combine Cells Into A Cell With Comma, Space and Vice VersaClifford Marco ArimadoNo ratings yet
- Citroen C4 Picasso/Grand Picasso BilmetropolenDocument5 pagesCitroen C4 Picasso/Grand Picasso BilmetropolenAlberto Miglino100% (1)
- Job - Details - Grant Acquisition Management (GAM) Manager - 5219Document4 pagesJob - Details - Grant Acquisition Management (GAM) Manager - 5219Salman DigaleNo ratings yet
- ConclusionDocument1 pageConclusionSAVITHRINo ratings yet
- Plaintiff's Original Petition: Uber Ridesharing DefendantDocument8 pagesPlaintiff's Original Petition: Uber Ridesharing DefendantWigingtonRumleyDunnBlairLLPNo ratings yet
- Plan Test Strategy for Flight Search WebsiteDocument13 pagesPlan Test Strategy for Flight Search WebsiteНаталья ПримаNo ratings yet
- HW1 Max Profit ProductionDocument7 pagesHW1 Max Profit ProductionTibelchNo ratings yet
- Guide Spec DX Air Outdoor Condensing Unit 2 2017Document5 pagesGuide Spec DX Air Outdoor Condensing Unit 2 2017JamesNo ratings yet
- Carbozinc 11Document4 pagesCarbozinc 11DuongthithuydungNo ratings yet
- A Comparative Analysis of Performance of Public & Private Sector Mutual FundsDocument30 pagesA Comparative Analysis of Performance of Public & Private Sector Mutual Fundsk kNo ratings yet
- State Board of Education Memo On Broward County (Oct. 4, 2021)Document40 pagesState Board of Education Memo On Broward County (Oct. 4, 2021)David SeligNo ratings yet
- Chapter 3 - Excel SolutionsDocument8 pagesChapter 3 - Excel SolutionsHalt DougNo ratings yet
- 20171121hulten JBS 6-3 PDFDocument13 pages20171121hulten JBS 6-3 PDFJe RomeNo ratings yet
- Forms6i 10GDocument42 pagesForms6i 10GRolando OcañaNo ratings yet
- 2019Document164 pages2019Nguyễn Sơn CaNo ratings yet
- Course Catalog 2021 2021 03 08Document14 pagesCourse Catalog 2021 2021 03 08hamzaNo ratings yet