Professional Documents
Culture Documents
PRISMA 2009 Flow Diagram: For More Information, Visit
Uploaded by
ManJ_mch0 ratings0% found this document useful (0 votes)
20 views1 pageOriginal Title
PRISMA 2009 flow diagram
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
20 views1 pagePRISMA 2009 Flow Diagram: For More Information, Visit
Uploaded by
ManJ_mchCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
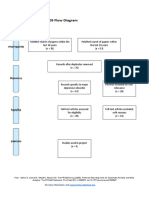
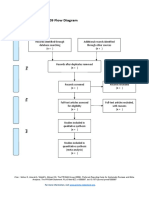
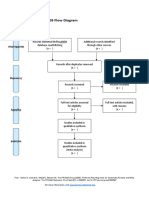
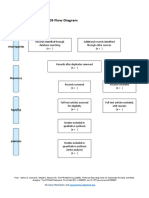
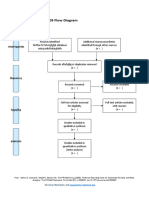
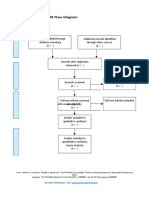
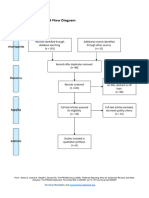
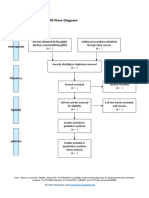
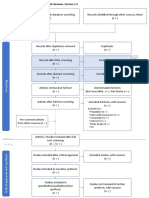
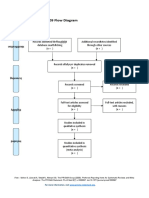
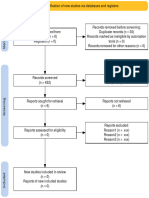
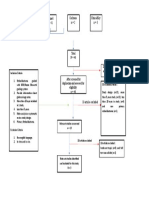
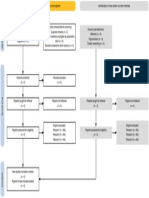
PRISMA 2009 Flow Diagram
Records identified through Additional records identified
Identification
database searching through other sources
(n = 34) (n = 0)
Records after duplicates removed
(n = 32)
Screening
Records excluded after
Records screened reviewing titles and
(n = 32) abstracts
(n = 12)
Full-text articles excluded,
with reasons
(n = 14)
Eligibility
Full-text articles assessed Not fulfilling selected
for eligibility outcome measures (n = 10)
Included other neurological
(n = 20)
disorders in subjects (n = 2)
Study focusing on Ankle
only (n = 1)
Not using Robotic devices
as experimental group
(n=1)
Included
Studies included in
systematic review
(n = 6)
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-
Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
For more information, visit www.prisma-statement.org.
You might also like
- Nonlinear Functional Analysis and Applications: Proceedings of an Advanced Seminar Conducted by the Mathematics Research Center, the University of Wisconsin, Madison, October 12-14, 1970From EverandNonlinear Functional Analysis and Applications: Proceedings of an Advanced Seminar Conducted by the Mathematics Research Center, the University of Wisconsin, Madison, October 12-14, 1970Louis B. RallNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitabdulNo ratings yet
- PRISMA 2009 Flow DiagramDocument1 pagePRISMA 2009 Flow DiagramAndrés Felipe Obregón HernándezNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepseNo ratings yet
- Green's Function Estimates for Lattice Schrödinger Operators and Applications. (AM-158)From EverandGreen's Function Estimates for Lattice Schrödinger Operators and Applications. (AM-158)No ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegututsgsgskhkhNo ratings yet
- PRISMA 2009 Flow Diagram GuideDocument1 pagePRISMA 2009 Flow Diagram GuideKou YashiroNo ratings yet
- PRISMA 2009 Flow Diagram GuideDocument1 pagePRISMA 2009 Flow Diagram GuidepedriyepsegututsgsgskhkhNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegututsgsgskhkhNo ratings yet
- PRISMA 2009 Flow Diagram MS WordDocument1 pagePRISMA 2009 Flow Diagram MS WordYuliza ArianiNo ratings yet
- PRISMA 2009 Flow Diagram PDFDocument1 pagePRISMA 2009 Flow Diagram PDFKalis WarenNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegututsgsgsNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegututsgsgskhkhNo ratings yet
- PRISMA 2009 Flow DiagramDocument1 pagePRISMA 2009 Flow DiagramnurulNo ratings yet
- PRISMA 2009 Flow Diagram PreenchidoDocument1 pagePRISMA 2009 Flow Diagram PreenchidoSDNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegsgsgsNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitpedriyepsegsgsgsNo ratings yet
- PRISMA 2020 Flow Diagram For Updated Systematic Reviews Which Included Searches of Databases, Registers and Other SourcesDocument1 pagePRISMA 2020 Flow Diagram For Updated Systematic Reviews Which Included Searches of Databases, Registers and Other SourcesSurya WijayaNo ratings yet
- PRISMA 2009 Flow DiagramDocument1 pagePRISMA 2009 Flow DiagrampedriyepsegsgsgsNo ratings yet
- PRISMA - 2020 - Flow - Diagram - Updated. JK Menambah Yg SDH Ada. TP Register OnlyDocument1 pagePRISMA - 2020 - Flow - Diagram - Updated. JK Menambah Yg SDH Ada. TP Register Onlyfachry albabNo ratings yet
- Flow ChartDocument2 pagesFlow Chartgeorgiana tangkilisanNo ratings yet
- Bmjopen 2020 December 10 12 Inline Supplementary Material 3Document1 pageBmjopen 2020 December 10 12 Inline Supplementary Material 3Alessandra Miranda PadillaNo ratings yet
- ROSES Flow Diagram For Systematic ReviewsDocument1 pageROSES Flow Diagram For Systematic Reviewswhy daNo ratings yet
- PRISMA 2009 Flow Diagram Guide for Systematic Literature ReviewsDocument1 pagePRISMA 2009 Flow Diagram Guide for Systematic Literature ReviewspedriyepseNo ratings yet
- PRISMA 2009 Flow Diagram PreenchidoDocument1 pagePRISMA 2009 Flow Diagram PreenchidoSDNo ratings yet
- PRISMA 2009 Flow Diagram IdentificationDocument1 pagePRISMA 2009 Flow Diagram IdentificationAndrés Felipe Obregón HernándezNo ratings yet
- PRISMA 2009 Flow DiagramDocument1 pagePRISMA 2009 Flow Diagrammoutasim mohammadNo ratings yet
- PRISMA 2009 Flow Diagram: 1. Youth, or Adolescents (N 100) 2. Irrelevant With Tubercolusi (N 35) 3Document1 pagePRISMA 2009 Flow Diagram: 1. Youth, or Adolescents (N 100) 2. Irrelevant With Tubercolusi (N 35) 3aisah andiniNo ratings yet
- PRISMA 2009 Flow Diagram PDFDocument1 pagePRISMA 2009 Flow Diagram PDFসোমনাথ মহাপাত্রNo ratings yet
- GROUP 5 PRISMA (Final)Document2 pagesGROUP 5 PRISMA (Final)Queen RosieNo ratings yet
- PRISMA 2009 Flow Diagram: For More Information, VisitDocument1 pagePRISMA 2009 Flow Diagram: For More Information, VisitRahma Aulia KhairunnisaNo ratings yet
- PRISMA 2009 Flow DiagramDocument1 pagePRISMA 2009 Flow DiagramsamiratumananNo ratings yet
- PRISMA Diagram TemplateDocument1 pagePRISMA Diagram TemplateRitashree DasguptaNo ratings yet
- ROSES Flow Diagram For Systematic MapsDocument1 pageROSES Flow Diagram For Systematic MapsLina DiazNo ratings yet
- ROSES Flow Diagram For Systematic MapsDocument1 pageROSES Flow Diagram For Systematic MapsJoanne WongNo ratings yet
- PRISMA 2020 Flow Diagram For New Systematic Reviews Which Included Searches of Databases, Registers and Other SourcesDocument1 pagePRISMA 2020 Flow Diagram For New Systematic Reviews Which Included Searches of Databases, Registers and Other SourcesSurya WijayaNo ratings yet
- NEW - Konvalesen Plasma - PRISMA 2009 Flow DiagramDocument1 pageNEW - Konvalesen Plasma - PRISMA 2009 Flow DiagramReynardi SutantoNo ratings yet
- PRISMA 2020 Flow Diagram New SRs v1Document1 pagePRISMA 2020 Flow Diagram New SRs v1eclipsesolidNo ratings yet
- PRISMA 2020 Flow Diagram New SRs v2Document1 pagePRISMA 2020 Flow Diagram New SRs v2Ivan PradhanaNo ratings yet
- PRISMA Flow DiagramDocument1 pagePRISMA Flow DiagramavfNo ratings yet
- PRISMA 2020 Flow Diagram New SRs v1Document1 pagePRISMA 2020 Flow Diagram New SRs v1Krisna ayuNo ratings yet
- PRISMA Italian Flow DiagramDocument1 pagePRISMA Italian Flow DiagramNathalia Arévalo NavarroNo ratings yet
- PRISMA 2020 Flow Diagram New SRs v1Document1 pagePRISMA 2020 Flow Diagram New SRs v1Rafael Calleja LozanoNo ratings yet
- PRISMA 2020 Flow Diagram SimpleDocument1 pagePRISMA 2020 Flow Diagram Simplecallytedford5No ratings yet
- PrismaDocument1 pagePrismaShanna FamaloanNo ratings yet
- Search StrategyDocument2 pagesSearch StrategyResearch Assistant of Dr. Rizal HamidNo ratings yet
- PRISMA - Urban e KhouryDocument1 pagePRISMA - Urban e KhourypasqualiniericaNo ratings yet
- Bagan Abstrak IACDocument1 pageBagan Abstrak IACJessica OtniellaNo ratings yet
- Prisma ChartDocument1 pagePrisma ChartHusnul FikriNo ratings yet
- PRISMA 2020 Flow Diagram New SRs v1Document1 pagePRISMA 2020 Flow Diagram New SRs v1Research and Academic AMSA-BrawijayaNo ratings yet
- PRISMA 2020 Flow Diagram New SRs v1-3Document1 pagePRISMA 2020 Flow Diagram New SRs v1-3crocs1086No ratings yet
- Clinical Effectiveness of Chin Cup Treatment For TDocument14 pagesClinical Effectiveness of Chin Cup Treatment For TIlzze Zuñigha100% (1)
- Prisma PediculosisDocument1 pagePrisma PediculosisAni TikaNo ratings yet
- PRISMA Rev3Document1 pagePRISMA Rev3Gosia StarzynskaNo ratings yet
- PrismaDocument1 pagePrismaheike silva garciaNo ratings yet
- PRISMA Flow Diagram Self Care Chronic Kidney DiseaseDocument1 pagePRISMA Flow Diagram Self Care Chronic Kidney DiseaseimranNo ratings yet
- Prisma PediculosisDocument1 pagePrisma PediculosisAni TikaNo ratings yet
- PRISMA 2020 Flow Diagram For New Systematic Reviews Which Included Searches of Databases and Registers OnlyDocument2 pagesPRISMA 2020 Flow Diagram For New Systematic Reviews Which Included Searches of Databases and Registers OnlyJavier Gio AlvarezNo ratings yet
- Gerund Infinitive Reduction 3Document1 pageGerund Infinitive Reduction 3Sefa KocaturkNo ratings yet
- Easy M Care ServicesDocument1 pageEasy M Care ServiceseasymNo ratings yet
- Roughdraft AllenDocument5 pagesRoughdraft Allenapi-318349384No ratings yet
- Jurnal NilamDocument7 pagesJurnal NilamSofia ChaeroniNo ratings yet
- Biometrics Authentication and Its ReliabilityDocument33 pagesBiometrics Authentication and Its ReliabilityAbhishek PpNo ratings yet
- Bulletin: Schema TherapyDocument16 pagesBulletin: Schema TherapyRafael CalpenaNo ratings yet
- Half Marathon Training Schedule in 12 WeeksDocument2 pagesHalf Marathon Training Schedule in 12 WeeksTose MomentNo ratings yet
- MD II Material Pentru 05.05.2020Document3 pagesMD II Material Pentru 05.05.2020Irina Panciu StefanNo ratings yet
- Psychological AssessmentDocument46 pagesPsychological Assessmentyubarajbro9104No ratings yet
- Modern TechnologyDocument1 pageModern Technologymanas reddyNo ratings yet
- Reflection - History of Philippine NursingDocument1 pageReflection - History of Philippine NursingCristelle EbolNo ratings yet
- Kudori Therapy - Updated 9th Dec17Document32 pagesKudori Therapy - Updated 9th Dec17Manickavasagam RengarajuNo ratings yet
- THEORIES OF ADDICTIONDocument24 pagesTHEORIES OF ADDICTIONManali NaphadeNo ratings yet
- Walmart Drug ListDocument6 pagesWalmart Drug ListShirley Pigott MDNo ratings yet
- CHN - Community Health Nursing - Lecture 1 (Prelim)Document4 pagesCHN - Community Health Nursing - Lecture 1 (Prelim)Akasha FrostmourneNo ratings yet
- NCM 104 Lecture Chapter 5 - NCDDocument39 pagesNCM 104 Lecture Chapter 5 - NCDWilma Nierva BeraldeNo ratings yet
- Guidelines For Beginning and Maintaining A Toy LendingDocument6 pagesGuidelines For Beginning and Maintaining A Toy LendingNurul HidayahNo ratings yet
- Pemahaman Tentang PCCDocument35 pagesPemahaman Tentang PCCBayu AdiputraNo ratings yet
- Jane Dunlap - Exploring Inner Space - Personal Experiences Under LSD-25 PDFDocument224 pagesJane Dunlap - Exploring Inner Space - Personal Experiences Under LSD-25 PDFJimy Robayo100% (1)
- Cryptosporidium Infection: Epidemiology, Pathogenesis, and Differential DiagnosisDocument5 pagesCryptosporidium Infection: Epidemiology, Pathogenesis, and Differential Diagnosismgch99No ratings yet
- Predictors ATLS FailureDocument6 pagesPredictors ATLS FailureKing DonNo ratings yet
- Spine TricksDocument345 pagesSpine Trickslacho124No ratings yet
- Ki Fact Sheet - FLDocument3 pagesKi Fact Sheet - FLvitruviuzNo ratings yet
- Risk For Compromised Human Dignity NCPDocument3 pagesRisk For Compromised Human Dignity NCPMarife Lipana Reyes100% (3)
- Chapter 1Document15 pagesChapter 1ErikaNo ratings yet
- Fphar 12 731201Document12 pagesFphar 12 731201I Made AryanaNo ratings yet
- Supplier Audit ChecklistDocument11 pagesSupplier Audit ChecklistOlexei Smart100% (1)
- Language Disorders: Causes, Symptoms and TreatmentDocument2 pagesLanguage Disorders: Causes, Symptoms and TreatmentLovielyn TubogNo ratings yet
- JMS - Filling Up New GravelDocument6 pagesJMS - Filling Up New GravelSilver SelwayneNo ratings yet
- Sprayspheres SC (SC BL Ve 1103 S)Document3 pagesSprayspheres SC (SC BL Ve 1103 S)asetaldehit yıldırımNo ratings yet