Professional Documents
Culture Documents
Cainta Catholic College: Daily Learning Activity Sheet
Uploaded by
Chynna Alicia B. EsguerraOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cainta Catholic College: Daily Learning Activity Sheet
Uploaded by
Chynna Alicia B. EsguerraCopyright:
Available Formats
Cainta Catholic College
A Bonifacio Ave. Cainta, Rizal
DAILY LEARNING ACTIVITY SHEET
Name: ____________________ Score: ______________________________
Year & Section: _____________ Day & Date Accomplished: ______________
Subject: Physical Science Parent/ Guardian Signature:_____________
_____________________________________________________________________________________
Activity Title: Isotopes and Mass Number
Learning Targets:
1. Explain how isotopes are formed
2. Determine atomic number and atomic mass of elements through subatomic particles.
3. Discuss the significance of subatomic particles in the study and formation of isotopes.
References: Fundamentals of Physical Science, Exploring Life Through Science Series
Author: Drexel H. Camacho, et al., Karen S. Santiago, et al. Page No: ________________
______________________________________________________________________________
Isotopes – refers to atoms with the same atomic number but different atomic masses.
The atomic number (Z) indicates the number of protons in an atom. In a neutral atom, the
number of protons is equal to the number of electrons. The atomic mass (A) is equal to the sum
of the number of protons and neutrons.
Example:
Isotopes of Hydrogen Number of protons Number of neutrons
protium 1 0
deuterium 1 1
tritium 1 2
Direction: Using the periodic table, answer the following questions.
1. How many protons are there in a chlorine atom? _____17_____
2. Which element has 20 protons and 20 neutrons? ___Calcium__
3. What is the atomic mass of an element that has an atomic number of 13? _____27_____
4. How many neutrons are there in a cobalt atom? _____33_____
5. How many electrons are there in a manganese atom? _____12_____
6. Which element has 15 protons and 15 electrons? __Phosphorus__
7. Find the atomic mass of an element with 19 protons and 20 neutrons. _____39_____
8. Which element has 4 protons and 5 neutrons? __Beryllium__
9. How many protons are there in an atom that has an atomic number of 30? _____30_____
10. How many electrons are there in a bromine atom? ______35______
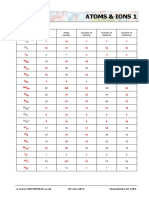
Direction: Complete the table below by giving the missing information about the following
elements.
Element Atomic Mass Number of Number of Number of
Number Number Protons Electrons Neutrons
Mg 12 24 12 12 12
K 19 39 19 19 20
Mn 25 55 25 25 30
Fe 26 56 26 26 30
Cu 29 64 29 29 35
Si 14 28 14 14 14
Kr 36 84 36 36 48
Ge 32 73 32 32 41
Ne 10 20 10 10 10
N 7 14 7 7 7
Ca 20 40 20 20 20
Br 35 80 35 35 45
P 15 31 15 15 16
Ar 18 40 18 18 22
Cr 24 52 24 24 28
You might also like
- Kelapa GadingDocument2 pagesKelapa GadingUtari Ika CahyaniNo ratings yet
- Chem Homework (2 Pages in Total) Structure of Atoms and IonsDocument2 pagesChem Homework (2 Pages in Total) Structure of Atoms and IonsAlbert YanNo ratings yet
- CHM ART Activity 4 Atoms and Isotopes PDFDocument4 pagesCHM ART Activity 4 Atoms and Isotopes PDFMark Vincent DoriaNo ratings yet
- Counting Subatomic Particles and Calculating Average Atomic Mass AssignmentDocument3 pagesCounting Subatomic Particles and Calculating Average Atomic Mass AssignmentDamien WhitakerNo ratings yet
- Summative Atomic StructureDocument3 pagesSummative Atomic StructureNovie Mae ReambonanzaNo ratings yet
- 01 - Atomic Theory Notes 2012 - KeyDocument2 pages01 - Atomic Theory Notes 2012 - Keyapi-292000448No ratings yet
- Basic Atomic Structure WorksheetDocument2 pagesBasic Atomic Structure WorksheetStephen100% (1)
- Basic Atomic Structure WorksheetDocument2 pagesBasic Atomic Structure WorksheetRhyz Mareschal DongonNo ratings yet
- Atomic StructureDocument2 pagesAtomic Structurephantuonglam3025No ratings yet
- Atomic Structure and Periodic Table PDFDocument51 pagesAtomic Structure and Periodic Table PDFKevin NdanyiNo ratings yet
- CH 2Document14 pagesCH 2dwarriorsNo ratings yet
- Chemsheets Atoms and IonsDocument1 pageChemsheets Atoms and IonsTommy CuninghameNo ratings yet
- Stretch SheetDocument2 pagesStretch Sheetjoe bloggNo ratings yet
- HL1-Chapter 2 Review SheetDocument5 pagesHL1-Chapter 2 Review SheetShafika AnuarNo ratings yet
- Sri Chaitanya: IIT Academy.,IndiaDocument10 pagesSri Chaitanya: IIT Academy.,Indiakarmanyab147No ratings yet
- Periodic Table Atomic NumbersDocument1 pagePeriodic Table Atomic NumbersPoojitha PuppalaNo ratings yet
- 9th Part 3Document2 pages9th Part 3Alwin S DavidNo ratings yet
- S1.3.3-1.3.5 Electron Configuration I MS FinalDocument4 pagesS1.3.3-1.3.5 Electron Configuration I MS FinalStruggl1ngNo ratings yet
- Atoms & Ions Worksheet 1 /63: Atomic Number and Mass NumberDocument4 pagesAtoms & Ions Worksheet 1 /63: Atomic Number and Mass Numbercate christineNo ratings yet
- Listening + Reading WorksheetDocument2 pagesListening + Reading WorksheetOnionNo ratings yet
- Atomic Structure 1Document27 pagesAtomic Structure 1Mamdooh AlqathamiNo ratings yet
- KCSE Form 2 NotesDocument139 pagesKCSE Form 2 NotesN KatanaNo ratings yet
- 121 Exam1 2009 KeyDocument8 pages121 Exam1 2009 KeyRicky WooNo ratings yet
- CHP 3 - Atomic Structure & The Periodic Table 1 QPDocument8 pagesCHP 3 - Atomic Structure & The Periodic Table 1 QPDhrumeelNo ratings yet
- CES Grade 2 Report Aralin Ang AlpabetoDocument6 pagesCES Grade 2 Report Aralin Ang AlpabetoGhie DomingoNo ratings yet
- SDFSFDocument3 pagesSDFSFAmyNo ratings yet
- Solution Manual For Chemistry 11th Edition by Chang ISBN 007766695X 9780077666958Document36 pagesSolution Manual For Chemistry 11th Edition by Chang ISBN 007766695X 9780077666958henryarmstrongypajbizoqe100% (23)
- Instrumental Neutron Activation Analysis (INAA)Document25 pagesInstrumental Neutron Activation Analysis (INAA)tibisavaNo ratings yet
- Worksheet 4Document2 pagesWorksheet 4Joan RosanaNo ratings yet
- All About The Periodic Table - Home Laboratory WorksheetDocument4 pagesAll About The Periodic Table - Home Laboratory WorksheetFrank Ed SerranoNo ratings yet
- Ions Practice - WKSTDocument2 pagesIons Practice - WKSTValerie ChappleNo ratings yet
- Worksheet 2 - Electrons Protons and NeutronsDocument3 pagesWorksheet 2 - Electrons Protons and NeutronsAnonymous ANo ratings yet
- Isotopes WS ANSWERS 1lmscf1Document1 pageIsotopes WS ANSWERS 1lmscf1team TSOTARENo ratings yet
- Science8 Q3 Week6Document20 pagesScience8 Q3 Week6Kathrina De SenaNo ratings yet
- Solution Manual For Chemistry 11Th Edition by Chang Isbn 007766695X 9780077666958 Full Chapter PDFDocument36 pagesSolution Manual For Chemistry 11Th Edition by Chang Isbn 007766695X 9780077666958 Full Chapter PDFtiffany.kunst387100% (10)
- Kami Export - Sajia Ehsani - Atomic Structure & The Periodic Table 1 QPDocument8 pagesKami Export - Sajia Ehsani - Atomic Structure & The Periodic Table 1 QPSajia EhsaniNo ratings yet
- INJSO2018 QuestionDocument20 pagesINJSO2018 QuestionmadhavNo ratings yet
- History and Subatomic Particle Review Take Two KEYDocument5 pagesHistory and Subatomic Particle Review Take Two KEYAlliya DaymonNo ratings yet
- Atom QuizDocument1 pageAtom QuizRose Ann BernardoNo ratings yet
- WEEK 2 Activity 2 ATOMIC VIEW OF MATTERDocument3 pagesWEEK 2 Activity 2 ATOMIC VIEW OF MATTERJim Jacob MotolNo ratings yet
- Name: - Class: 3 - DateDocument3 pagesName: - Class: 3 - DateokNo ratings yet
- Paper 1 HarshalDocument6 pagesPaper 1 HarshalHarshal MadaviNo ratings yet
- DILR LOD2 25th August 2022 Batch 1 NotesDocument4 pagesDILR LOD2 25th August 2022 Batch 1 NotesMohtasim khanNo ratings yet
- VILLONDO Atomic Number, Mass Number and IsotopesDocument7 pagesVILLONDO Atomic Number, Mass Number and IsotopesTito V. Bautista Jr.No ratings yet
- Kami Export - Arabella McCarthy - Unit 3 Atom Packet - 10!04!2017!12!20 - 25 - 057Document4 pagesKami Export - Arabella McCarthy - Unit 3 Atom Packet - 10!04!2017!12!20 - 25 - 057Arabella McCarthyNo ratings yet
- Naa 1 (HW)Document3 pagesNaa 1 (HW)Kissiedu YirenkyiNo ratings yet
- Exerciese 5.1. The Electron Arrangement of Elements With Proton Number 1 To 20. Number of Valence ElectronsDocument2 pagesExerciese 5.1. The Electron Arrangement of Elements With Proton Number 1 To 20. Number of Valence ElectronsZarina IdrisNo ratings yet
- Geochimie Curs 1Document5 pagesGeochimie Curs 1Istrate IsabelaNo ratings yet
- Electron Config WSDocument3 pagesElectron Config WSCel blazNo ratings yet
- SLG 5.3.1 Isotopes, Isotones, IsobarsDocument7 pagesSLG 5.3.1 Isotopes, Isotones, IsobarsPaul CustodioNo ratings yet
- Basic Atomic Structure WorksheetDocument4 pagesBasic Atomic Structure WorksheetTrisha GolesNo ratings yet
- Ultimo Horario 2023Document2 pagesUltimo Horario 2023Rodrigo Pancorbo VillaNo ratings yet
- Atomic Structure AnswerDocument8 pagesAtomic Structure Answer6brk8sjszqNo ratings yet
- Sri Chaitanya IIT Academy., India: ChemistryDocument18 pagesSri Chaitanya IIT Academy., India: ChemistryM jhansiNo ratings yet
- Final Result Fybcom Sem I (Nep)Document201 pagesFinal Result Fybcom Sem I (Nep)hussainpalanpurwalaoffers53No ratings yet
- Yeni Tugasmk Off DDocument4 pagesYeni Tugasmk Off Dyeni maratussNo ratings yet
- Electronegativity WorksheetDocument1 pageElectronegativity WorksheetLucita P. CatarajaNo ratings yet
- Crossword Sc1Document2 pagesCrossword Sc1juanx30No ratings yet
- Chapter 1 - Atomic Structure: Test Yourself (Page 3)Document139 pagesChapter 1 - Atomic Structure: Test Yourself (Page 3)Aref DahabrahNo ratings yet
- FINAL Project in DISS Activity 2: Story Maker Time!Document4 pagesFINAL Project in DISS Activity 2: Story Maker Time!Chynna Alicia B. EsguerraNo ratings yet
- DocumentDocument1 pageDocumentChynna Alicia B. EsguerraNo ratings yet
- The Name Chynna Means "The Spirit of The Ancient Ocean"Document1 pageThe Name Chynna Means "The Spirit of The Ancient Ocean"Chynna Alicia B. EsguerraNo ratings yet
- DocumentDocument2 pagesDocumentChynna Alicia B. EsguerraNo ratings yet
- My School Life During Covid-19Document1 pageMy School Life During Covid-19Chynna Alicia B. EsguerraNo ratings yet
- Quiz 2Document3 pagesQuiz 2Chynna Alicia B. EsguerraNo ratings yet
- DocumentDocument1 pageDocumentChynna Alicia B. EsguerraNo ratings yet
- Task Objective: Task Objective: Task Objective:: Highschool LevelDocument3 pagesTask Objective: Task Objective: Task Objective:: Highschool LevelChynna Alicia B. EsguerraNo ratings yet
- Chapter 4 - Wilhelm Wundt and The Founding of Psychology: Dr. Nancy AlvaradoDocument27 pagesChapter 4 - Wilhelm Wundt and The Founding of Psychology: Dr. Nancy AlvaradoChynna Alicia B. EsguerraNo ratings yet
- Cainta Catholic College: High School Department A.Y 2019-2020 Course Outline Earth and Life ScienceDocument2 pagesCainta Catholic College: High School Department A.Y 2019-2020 Course Outline Earth and Life ScienceChynna Alicia B. EsguerraNo ratings yet
- Color LinesDocument8 pagesColor LinesChynna Alicia B. EsguerraNo ratings yet
- 2.9. Commission On Independence, Filipino Grievances Against Governor Wood (Zaide, 11. Pp. 230-234) - (Petition Letter) Historical BackgroundDocument5 pages2.9. Commission On Independence, Filipino Grievances Against Governor Wood (Zaide, 11. Pp. 230-234) - (Petition Letter) Historical BackgroundChynna Alicia B. EsguerraNo ratings yet
- Class 6 Physics: (Lesson Details For Final Exams) : Syllabus: Chapter 2.07, 2.08 and 2.10Document2 pagesClass 6 Physics: (Lesson Details For Final Exams) : Syllabus: Chapter 2.07, 2.08 and 2.10tasnimNo ratings yet
- Labrotary Work 1Document4 pagesLabrotary Work 1kevin loachaminNo ratings yet
- First Physics Monthly Exam Second TrimesterDocument2 pagesFirst Physics Monthly Exam Second TrimesterGabriel FigueroaNo ratings yet
- Criminilistic v2.0 NEED To KNOW!Document376 pagesCriminilistic v2.0 NEED To KNOW!jushuasalingayNo ratings yet
- Relative Dielectric Permittivity in Porous MediaDocument10 pagesRelative Dielectric Permittivity in Porous MediaLazuardhy Vozika FuturNo ratings yet
- Natural Snow BuildingsDocument17 pagesNatural Snow BuildingsStrannik RuskiNo ratings yet
- HA-SERIES OPERATION MANUAL 02ver PDFDocument354 pagesHA-SERIES OPERATION MANUAL 02ver PDFsunhuynhNo ratings yet
- CaseStudyQs-12th-FREE DEMODocument10 pagesCaseStudyQs-12th-FREE DEMOSaloni GuptaNo ratings yet
- Week 4 Combined - AER Feb 24Document19 pagesWeek 4 Combined - AER Feb 24Omkar BhavsarNo ratings yet
- 2017 Sec3 EMath NA 12sDocument407 pages2017 Sec3 EMath NA 12sdanny hernandezNo ratings yet
- Intro To Ultrasonics Edit1Document46 pagesIntro To Ultrasonics Edit1Asep WahyuNo ratings yet
- Heat 1Document36 pagesHeat 1ZainabNo ratings yet
- Lecture 25 - Steady-State Behavior of Markov ChainsDocument10 pagesLecture 25 - Steady-State Behavior of Markov ChainsK VirtuosoNo ratings yet
- Cilamce 2013 - 0690Document20 pagesCilamce 2013 - 0690José Guilherme Santos da SilvaNo ratings yet
- Grade 5 - Arts ActivitiesDocument7 pagesGrade 5 - Arts ActivitiesMarcus Sayson DabonNo ratings yet
- Problemset 1Document3 pagesProblemset 1Pratap KunathiNo ratings yet
- CO1 - Problems - Magnetic PropertiesDocument8 pagesCO1 - Problems - Magnetic Propertieskowshik ReddyNo ratings yet
- PHME16 CM Bearing FinalDocument18 pagesPHME16 CM Bearing Finalowais khanNo ratings yet
- Journal of Food Engineering: Matteo Cordioli, Massimiliano Rinaldi, Gabriele Copelli, Paolo Casoli, Davide BarbantiDocument8 pagesJournal of Food Engineering: Matteo Cordioli, Massimiliano Rinaldi, Gabriele Copelli, Paolo Casoli, Davide Barbantimohammad9906426240No ratings yet
- EE 312 - Exp2 - Slides - NDocument31 pagesEE 312 - Exp2 - Slides - Nعبدالرحمن النويصرNo ratings yet
- Dam Deformation-2Document20 pagesDam Deformation-2Fajar A KurniawanNo ratings yet
- Chapter 15 (Read-Only)Document35 pagesChapter 15 (Read-Only)Mai Anh ThưNo ratings yet
- Distance Learning Programme: Jee (Main) : Leader Test Series / Joint Package CourseDocument8 pagesDistance Learning Programme: Jee (Main) : Leader Test Series / Joint Package CourseAnupam SinghNo ratings yet
- Productattachments Files e - E-T-Humidefs 10 PDFDocument4 pagesProductattachments Files e - E-T-Humidefs 10 PDFNeelakandan DNo ratings yet
- Cepheus Journal Issue 007bDocument40 pagesCepheus Journal Issue 007bScott Tay100% (1)
- Lesson #15 Centroid of Plane Areas and of Solids of Revolution-1Document20 pagesLesson #15 Centroid of Plane Areas and of Solids of Revolution-1yveeshNo ratings yet
- 4.222 S.y.b.sc - Physics Sem III IVDocument24 pages4.222 S.y.b.sc - Physics Sem III IVChandan GuptaNo ratings yet
- Lesson Plan - Atomic Structure (SEMI DETAILED PLAN)Document3 pagesLesson Plan - Atomic Structure (SEMI DETAILED PLAN)Rjane Cañete89% (9)
- Iit RPR Ma201Document2 pagesIit RPR Ma201atggregNo ratings yet
- Design of Experiments - Week 1, 2Document50 pagesDesign of Experiments - Week 1, 2Akram KhanNo ratings yet