Professional Documents
Culture Documents
Chapter 1 CHM 207
Uploaded by
MIZUKI JIROOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 1 CHM 207
Uploaded by
MIZUKI JIROCopyright:
Available Formats
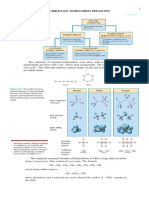
ISOMERISM
ISOMERS: ORGANIC COMPOUNDS THAT HAVE SAME MOLECULAR FORMULA BUT
DIFFERENT ARRANGEMENTS OF ATOMS.
ISOMERISM: THE EXISTENCE OF TWO OR MORE ORGANIC COMPOUNDS WITH THE
SAME MOLECULAR FORMULA BUT DIFFERENT ARRANGEMENTS OF ATOMS.
STRUCTURAL ISOMERISM: SAME MOLECULAR ISOMER BUT DIFFERENT STRUCTURAL
ORGANIC COMPOUND – ONE THAT CARBON AS PRINCIPLE FORMULA.
ELEMENT. STEREOISOMERISM: SAME STRUCTURAL FORMULA BUT THEY HAVE DIFFERENT
HYDROCARBON – CONTAIN ONLY C & H SPATIAL ARRANGEMENTS
FUNCTIONAL GROUP- ATOMS, GROUP OF ATOMS OR BOND REACTION OF ORGANIC COMPOUNDS
THAT DETERMINE THE CHEMICAL PROPERTIES OF ORGANIC · CARBOCATION IS A MOLECULE THAT
COMPOUNDS. CARRY POSITIVE CHARGE ON CARBON
HOMOLOGOUS SERIES - GROUP OF COMPOUNDS WITH THE ATOM
SAME FUNCTIONAL GROUPS. · FREE RADICAL IS A REACTIVE ATOM OR
GROUP OF ATOMS THAT HAS ONE OR
INTRODUCTION TO MORE UNPAIRED ELECTRONS
· > R GROUP > STABILITY
ORGANIC CHEMISTRY
ELECTROPHILES AND NUCLEOPHILES :
ELECTROPHILES
ELECTRON-LOVING
ACCEPT ELECTRON
+VE OR NEUTRAL CHARGE
BONDING HYBRIDIZATION THEORY STRUCTURAL FORMULA
LEWIS ACIDS
ATOM BOND TOGETHER TO BECOME MORE STABLE. EXPANDED
NEUTROPHILE
VALENCE BOND THEORY CONDENSED
NUCLEUS LOVING
COVALENT BONDS - ELECTRONS PAIRED IN THE OVERLAPPING ORBITALS SKELETAL
DONATE ELECTRON
SIGMA BOND (Σ) & PI BOND (Π) FISHER PROJECTION
-VE OR NEUTRAL CHARGE
HYBRIDIZATION: COMBINATION OF TWO OR MORE ATOMIC ORBITALS TO FORM THE
LEWIS BASE
SAME NUMBER OF HYBRID ORBITALS.
THREE TYPES OF HYBRIDIZATION
SP – TRIPLE BOND (1Σ AND 2Π BOND)
SP2 – DOUBLE BOND (1Σ AND 1Π BOND)
SP3 – SINGLE BOND (1Σ)
You might also like
- Chemical Processes ExplainedDocument78 pagesChemical Processes ExplainedEmier VillanuevaNo ratings yet
- Introduction To Organic ChemistryDocument29 pagesIntroduction To Organic ChemistryalinNo ratings yet
- Isomerism 1Document66 pagesIsomerism 1Yaaminni ArumukamNo ratings yet
- Part1 StereochemistryDocument134 pagesPart1 StereochemistryOrganic ChemistryNo ratings yet
- BSPH-1101 Resonance Structure ORGANIC Chemistry Le: Ms. Krishally Joy O. Patalinjug - Feb. 2022Document3 pagesBSPH-1101 Resonance Structure ORGANIC Chemistry Le: Ms. Krishally Joy O. Patalinjug - Feb. 2022Orianna SanoNo ratings yet
- 3-d Structure Configuration & ConformationDocument13 pages3-d Structure Configuration & ConformationRon008No ratings yet
- CH3 Introduction of Organic Chemistry 20222023 P2Document31 pagesCH3 Introduction of Organic Chemistry 20222023 P2Daniel MukhrizNo ratings yet
- Introduction To Organic ChemistryDocument3 pagesIntroduction To Organic ChemistryDelosreyes ChildrenNo ratings yet
- Basic chemistry laws: Conservation, multiple proportions, gaseous volumesDocument1 pageBasic chemistry laws: Conservation, multiple proportions, gaseous volumesKadek Indah PuspaNo ratings yet
- Lesson 1 Introduction To Organic Chemistry PDFDocument4 pagesLesson 1 Introduction To Organic Chemistry PDFdela2No ratings yet
- Physics Organic ChemistryDocument8 pagesPhysics Organic Chemistrypmasingi.atworkNo ratings yet
- 2021-2022 - Isomers of HydrocarbonsDocument30 pages2021-2022 - Isomers of HydrocarbonsKevin KuaNo ratings yet
- IsomerismDocument30 pagesIsomerismTristan PereyNo ratings yet
- 2nd ReporterDocument51 pages2nd ReporterCedrick Earl D. GuhitiaNo ratings yet
- Chem 201/beauchamp Topic 7, Stereochemistry 1 Chem 201/beauchamp Topic 7, Stereochemistry 2Document13 pagesChem 201/beauchamp Topic 7, Stereochemistry 1 Chem 201/beauchamp Topic 7, Stereochemistry 2beatrice cho ming xuanNo ratings yet
- Adobe Scan Sep 04, 2023Document1 pageAdobe Scan Sep 04, 2023sagargupta8c.jsspNo ratings yet
- Part-1 Stereochemistry of Organic CompoundsDocument28 pagesPart-1 Stereochemistry of Organic CompoundsIct Pfa ClubNo ratings yet
- P11-13 Isomerisms Functional Groups and ReactionDocument62 pagesP11-13 Isomerisms Functional Groups and ReactionNing CahNo ratings yet
- Stereo Chemistry PDFDocument23 pagesStereo Chemistry PDFHarsh MewadaNo ratings yet
- IsomersDocument1 pageIsomersAnna LengyelNo ratings yet
- Mind Mapping Kimia KarbonDocument1 pageMind Mapping Kimia KarbonI Putu Adi Payana PutraNo ratings yet
- A New Fundamental Type of Conformational IsomerismDocument10 pagesA New Fundamental Type of Conformational IsomerismBakshi Agarwal PatelNo ratings yet
- Organic Chemistry Midterms Lecture NotesDocument9 pagesOrganic Chemistry Midterms Lecture NotesAira Lene ManaysayNo ratings yet
- Supramolecular ChemistryDocument52 pagesSupramolecular ChemistryShresth GuptaNo ratings yet
- Organic Unit-2Document22 pagesOrganic Unit-2Ashish MahereNo ratings yet
- Types of Organic IsomerismDocument1 pageTypes of Organic IsomerismBetty WeissNo ratings yet
- Dummy Dummy LectureDocument1 pageDummy Dummy LectureMark Cliffton BadlonNo ratings yet
- Chap 01 Some Basic Principles of Organic ChemistryDocument13 pagesChap 01 Some Basic Principles of Organic ChemistryParth JainNo ratings yet
- Aerobic and Anaerobic Respiration DiagramDocument1 pageAerobic and Anaerobic Respiration DiagramMica Angela AlteraNo ratings yet
- 10 1039@c5ob00173kDocument33 pages10 1039@c5ob00173kAlex FNo ratings yet
- Introduction To ChemistryDocument18 pagesIntroduction To ChemistryViktor Mikhael Roch BarbaNo ratings yet
- WEEK 2 INTRODUCTION TO ORGANIC CHEMISTRY Structure and BondingDocument7 pagesWEEK 2 INTRODUCTION TO ORGANIC CHEMISTRY Structure and BondingKyle Dennis SantosNo ratings yet
- Stereochemistry PDFDocument6 pagesStereochemistry PDFamrhkmhNo ratings yet
- Inorganic and Organic ChemistryDocument8 pagesInorganic and Organic ChemistryValerie BorrioNo ratings yet
- QSB 06 - Shape and Structure of ProteinsDocument29 pagesQSB 06 - Shape and Structure of Proteinsfta2013No ratings yet
- Chemistry of Carbon Compounds - Structure and FormulaeDocument53 pagesChemistry of Carbon Compounds - Structure and FormulaeTashane officialNo ratings yet
- Caps Organic ChemistryDocument56 pagesCaps Organic ChemistryIamThatoNo ratings yet
- AP Biology Unit 1: Biochemistry Cheat Sheet: by ViaDocument1 pageAP Biology Unit 1: Biochemistry Cheat Sheet: by Viamelia sabaNo ratings yet
- Reviewer Chem RemiDocument2 pagesReviewer Chem RemiBuena QuintinNo ratings yet
- Cell AnatomyDocument9 pagesCell AnatomykurisutineneNo ratings yet
- Classification, Nomenclature and Isomerism PDFDocument34 pagesClassification, Nomenclature and Isomerism PDFSantosh Potdar100% (4)
- Hydrocarbons and Hydrocarbon DerivativesDocument9 pagesHydrocarbons and Hydrocarbon DerivativesMark Robert MagsinoNo ratings yet
- Polymers 2021Document135 pagesPolymers 2021Roselyn CastilloNo ratings yet
- General Organic Chemistry: Most Important Questions & MindmapDocument368 pagesGeneral Organic Chemistry: Most Important Questions & MindmapmubarakaishmubbuNo ratings yet
- Year 12 Chemistry SOLDocument3 pagesYear 12 Chemistry SOLHansika SamudralaNo ratings yet
- Biomolecules and Cell Organelles LessonDocument2 pagesBiomolecules and Cell Organelles LessonRoahit RajanNo ratings yet
- IsomerismDocument4 pagesIsomerismBisha MonNo ratings yet
- Chem 111 LabDocument7 pagesChem 111 LabAira Lene ManaysayNo ratings yet
- Organic ChemistryDocument7 pagesOrganic ChemistryMariellaIsabelCasuyonNo ratings yet
- Def 1Document1 pageDef 1ZunairaSafdarNo ratings yet
- Combinepdf-7 CompressedDocument21 pagesCombinepdf-7 Compressedaichiii.bearNo ratings yet
- Chem Reviewer (Lec & Lab)Document9 pagesChem Reviewer (Lec & Lab)adelaine perasNo ratings yet
- BIOCHEMISTRY LEC Module 1Document8 pagesBIOCHEMISTRY LEC Module 156bmkkn2rnNo ratings yet
- Conjugated SystemDocument11 pagesConjugated SystemRajaAkmalNo ratings yet
- StereochemistryppDocument19 pagesStereochemistryppMohammad RussellNo ratings yet
- CARDIOVASCULAR SYSTEM BIOCHEMISTRY ENZYMESDocument5 pagesCARDIOVASCULAR SYSTEM BIOCHEMISTRY ENZYMESRose ImeeNo ratings yet
- BCCH 3Document79 pagesBCCH 3NG SIRNo ratings yet
- An Explanation of The Property of Induced Polarity of Atoms and An Interpretation of The Theory of Partial Valencies On An Electronic BasisDocument14 pagesAn Explanation of The Property of Induced Polarity of Atoms and An Interpretation of The Theory of Partial Valencies On An Electronic Basisluiz13eduardoNo ratings yet
- Covalent BondsDocument26 pagesCovalent BondsMabelle DucusinNo ratings yet
- Cellular Aspects of Membrane Permeability: International Series of Monographs in Pure and Applied Biology: Modern Trends in Physiological SciencesFrom EverandCellular Aspects of Membrane Permeability: International Series of Monographs in Pure and Applied Biology: Modern Trends in Physiological SciencesNo ratings yet
- Reaction Heats and Bond Strengths: Based on a Series of Lectures Given to Postgraduate Students at the University of Keele, 1960From EverandReaction Heats and Bond Strengths: Based on a Series of Lectures Given to Postgraduate Students at the University of Keele, 1960No ratings yet
- Vtu Previous Year Question PapersDocument26 pagesVtu Previous Year Question Papersprashanth prabhuNo ratings yet
- Corrosion of Iron NailsDocument2 pagesCorrosion of Iron Nailseun mee0% (1)
- Chirality, Carbonyls and Carboxylic Acids QuestionsDocument11 pagesChirality, Carbonyls and Carboxylic Acids QuestionsMohamed ZaidhanNo ratings yet
- General Science Capsule 2019Document26 pagesGeneral Science Capsule 2019Mazhar AliNo ratings yet
- Tech Talk Liquid Filtration Pressure DropDocument1 pageTech Talk Liquid Filtration Pressure DropAzmi AhmadNo ratings yet
- Physical Properties of Matter LessonDocument6 pagesPhysical Properties of Matter LessonKylene MontalbaNo ratings yet
- Biological Activity and Chemical Characterization of Pouteria LucumaDocument9 pagesBiological Activity and Chemical Characterization of Pouteria LucumaErnesto VilchezNo ratings yet
- MViQ UV Probe Spec Sheet BHCS33368Document2 pagesMViQ UV Probe Spec Sheet BHCS33368Jocélio A. TavaresNo ratings yet
- ExamDocument10 pagesExamEllen MarksNo ratings yet
- Cossack - Summary Crude Oil Assay Report: Source of Sample Light Hydrocarbon Analysis Assay Summary / TBP DataDocument55 pagesCossack - Summary Crude Oil Assay Report: Source of Sample Light Hydrocarbon Analysis Assay Summary / TBP DataDaniel LautaroNo ratings yet
- Science of The Egg Drop1Document2 pagesScience of The Egg Drop1Virginia FernandezNo ratings yet
- BSACIST 2019 Publications ListDocument263 pagesBSACIST 2019 Publications ListYaswanth SinhaNo ratings yet
- Citacores Teoria Gravitacao 1811.10556Document24 pagesCitacores Teoria Gravitacao 1811.10556Diretoria Sigma SocietyNo ratings yet
- Yuanchen PTFE Thread Raw Material MSDSDocument8 pagesYuanchen PTFE Thread Raw Material MSDSRene ArellanoNo ratings yet
- Sec 1 Science Mavis Mid Answer BookletDocument3 pagesSec 1 Science Mavis Mid Answer Bookletenna choyNo ratings yet
- Validation and Application of A Kinetic Model For Downdraft Biomass Gasification SimulationDocument28 pagesValidation and Application of A Kinetic Model For Downdraft Biomass Gasification SimulationMukiibi DuncanNo ratings yet
- CHEG 211 Chemical Process Calculation Homework #1Document2 pagesCHEG 211 Chemical Process Calculation Homework #1ramesh pokhrelNo ratings yet
- Production of Sucroesters Using Solvent-FreeDocument8 pagesProduction of Sucroesters Using Solvent-FreeAlfonso Dominguez GonzalezNo ratings yet
- D 5292 - 99 RduyotiDocument7 pagesD 5292 - 99 RduyotiRuben YoungNo ratings yet
- A Heterocylic (212 C Part 2)Document138 pagesA Heterocylic (212 C Part 2)Moamen MohamedNo ratings yet
- Lascaray1952 - INDUSTRIAL FAT SPLITTINGDocument5 pagesLascaray1952 - INDUSTRIAL FAT SPLITTINGPrabandari 632No ratings yet
- Standardized Test Prep Corrections and AnswersDocument3 pagesStandardized Test Prep Corrections and AnswersVicky LiNo ratings yet
- Semiconductor Electronics Class 12Document73 pagesSemiconductor Electronics Class 12sarkaraditya249No ratings yet
- Principles and Applications Of: PyrometallurgyDocument19 pagesPrinciples and Applications Of: PyrometallurgyChamel Jamora RuperezNo ratings yet
- 9Document11 pages9Andrea MolinaNo ratings yet
- Safety Data Sheet: Welding Torch - CoolantDocument8 pagesSafety Data Sheet: Welding Torch - CoolantDheebika MurugesanNo ratings yet
- Chapter 2 - Sample Problem SolutionDocument7 pagesChapter 2 - Sample Problem SolutiondiamantechennieNo ratings yet
- Phytochemical Analysis - 2001 - Mehta - Determination of Marker Constituents From Cissus Quadrangularis Linn and TheirDocument6 pagesPhytochemical Analysis - 2001 - Mehta - Determination of Marker Constituents From Cissus Quadrangularis Linn and TheirAna HeloisaNo ratings yet