Professional Documents
Culture Documents
Pressure Swing Adsorption in The Unit Operations Laboratory
Pressure Swing Adsorption in The Unit Operations Laboratory
Uploaded by
balasubramani krishnamurthiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pressure Swing Adsorption in The Unit Operations Laboratory
Pressure Swing Adsorption in The Unit Operations Laboratory
Uploaded by
balasubramani krishnamurthiCopyright:
Available Formats
ChE laboratory
PRESSURE SWING ADSORPTION IN
THE UNIT OPERATIONS LABORATORY
Jason C. Ganley
P
Colorado School of Mines • Golden, CO 80401
ressure swing adsorption (PSA) is an industrial process adsorption occurs. The selection of a particular type of ad-
typically used for the bulk separation of gas mixtures. sorbent may allow one component of a gas mixture to be
An outgrowth of temperature swing adsorption, PSA preferentially adsorbed, the nature of surface and/or pore dif-
is one of only a few gas-surface adsorption processes that fusion effects may vary for each gas in the mixture, and so on.
allows for the separation of mixtures of gaseous species or PSA is a cyclic operation, and generally involves separa-
vapors that exist in relatively high (non-trace) concentra- tion of a gas mixture by taking advantage of differences in
tions in respect to one another. PSA has also been coupled adsorption thermodynamics or in diffusion rates that exist for
to distillation processes for the separation of alcohol-water its various components. Cycling between higher and lower
vapor azeotropes.[1] pressures allows components of the gas mixture to be removed
It is interesting to note that, despite serving an important from (and later released to) the gas phase over designated pe-
role for many decades in the chemical process industry, PSA riods of time. Students in the Unit Operations Laboratory may
is not a central topic discussed in most chemical engineering consider the PSA cycle primarily as a mass transfer (rather
educational resources, which instead focus on mass transfer than heat transfer) experiment. The packed beds used in com-
unit operations. To the author’s knowledge, the present com- mercial PSA units are designed to be isothermal over an entire
munication represents the first reported incorporation of PSA cycle. That is, although there are usually large exotherms for
within an undergraduate unit operations laboratory. gas adsorption (and large endotherms for desorption), the
While PSA is commonly used for hydrogen and hydrocar- adsorption exotherms are used to provide the necessary heat
bon systems, it is also a popular process for the separation of for desorption of the same gases during pressure reduction
air into product streams of enriched nitrogen, or of enriched or purge steps later in the cycle. Additionally, large systems
oxygen. The process of air separation by PSA is an excel- usually have sufficient heat capacity (or thermal mass) so that
lent illustration of the principles of gas-solid mass transfer large temperature variations do not develop within the system.
within a unit operations laboratory course—there are no In this laboratory exercise, students use a custom-built,
harmful chemicals involved, the operational pressures are not four-column PSA system; one pair of columns contains carbon
excessively high, the effective gas concentrations are easily molecular sieve (CMS) adsorbent, and the other pair is filled
measured and distinguished, and the equipment lifetime is with 13X molecular sieve (sodium alumina-silicate). Students
practically unlimited (provided that the feed air is properly are able to vary a wide range of experimental parameters
filtered and dried). The physical and chemical phenomena within the system. These include, but are not limited to:
utilized in a PSA system are quite simple, and PSA units are • Adsorption pressure
usually designed to take advantage of one of two distinct
• Purge gas type, flow rate, and pressure
aspects.
1) Kinetic Control: In porous solids, there may be a differ-
ence in the rate at which various gases may diffuse to Jason Ganley is an associate teaching
and from regions of the solid surface. professor of chemical engineering at the
Colorado School of Mines. He earned a B.S.
2) Equilibrium Control: Over a range of partial pressures degree from the University of Missouri-Rolla
of species within a mixture of gases, there may be dif- and M.S. and Ph.D. degrees from the Uni-
fering equilibrium surface concentrations of adsorbed versity of Illinois at Urbana-Champaign, all in
chemical engineering. His current research
gases on solid materials that are exposed to the gas focuses on the production of alternative
mixture. fuels from renewable energy.
Therefore, the nature of the adsorbent material will strongly
influence the amount of adsorption and the speed at which
© Copyright ChE Division of ASEE 2018
44 Chemical Engineering Education
• Feed and product flow
rates/gas residence time
• Column flow configura-
tion/cycle style
A more detailed description
of the experimental apparatus
and its operation will follow.
INDUSTRIAL PRAC-
TICE AND LITERA-
TURE REVIEW
PSA was developed inde-
pendently by the Air Liq-
uide and Exxon corporations
in the 1960s. Since then, it
has proven to be a versa-
tile and effective process for
separation or purification of
many gas systems; high final
product purities are routinely
achieved. The application
of PSA to air separation can
produce nitrogen products of
99.9+% purity, and oxygen
product purities exceeding
95%.[2] Since its introduction
to the chemical process indus-
try, research and development
of PSA processes have led to
several significant process
improvements and variations,
most of which are simply tim-
ing or sequence modifications
to the earliest pressure swing
cycles.[3] The various cycles
and their modifications are
most easily illustrated in terms
of systems with two columns
packed with adsorbent. In
such a system, these columns
may be used independently,
or in tandem. Two of the more
common industrial cycles for
two-column systems are il-
lustrated in Figure 1.[4]
Figure 1 illustrates two dif-
ferent pressure swing cycles
with a two-column system.
Figure 1(b) does not illustrate
a direct interaction between
the two columns, although it
is worth noting that multiple Figure 1:Example
Figure 1. Example PSA
PSA cycles:
cycles: (a) backfill
(a) backfill cycle,
cycle, (b) (b)swing
vacuum vacuum swing cycle.
cycle.
Vol. 52, No. 1, Winter 2018 45
1
Figure 2.
PSA ex-
perimental
process
flow
schematic.
Measure-
ments
with an
asterisk (*)
indicate
sensors
with auto-
matically
logged
analog
outputs.
columns would be requiredFigure 2: PSA
to allow experimental
for continuous process Step
product flow3:schematic. Measurements
When the nitrogen purity of thewith an gas nears
product
asterisk (*) indicate sensors with automatically logged analog outputs.
collection. As an operational example, consider the backfill its lower allowed limit, the product release valve is closed.
cycle in Figure 1(a) as applied to nitrogen production. In this The feed to Column 1 and the purge valve for Column 2 are
case, the adsorption packing is chosen and used to preferen- both closed, and Column 2 is now charged with feed air while
tially hold up oxygen (rather than nitrogen) in the column dur- Column 1 is blown down.
ing the period of product collection. Below, a short description Step 4: Column 2 provides backfilling to Column 1 while
of each process step in the cycle is described, along with an generating more nitrogen-rich product gas until the minimum
associated statement of gas/surface interaction. product purity is once again reached. Closing the product,
Step 1: Freshly purged and at a low (e.g., atmospheric) pres- feed, and purge valves once again resets the system to the
sure, Column 1 is quickly pressurized with an air feed stream cycle’s beginning (Step 1).
to the system’s selected working pressure. Simultaneously, Industrial pressure swing adsorption systems operate by
Column 2 is saturated with adsorbed oxygen at the work- continuously repeating such cycle steps, executing them with
ing pressure, and is vented to the atmosphere (blowdown). automated valves and some form of integrated product gas
In Column 1, the adsorption packing quickly fixes oxygen storage.[5] Typical systems may take many complete cycles
onto its surface, allowing proportionally more nitrogen to to reach a round trip steady-state operation[6,7] as well. The
remain in the gas phase. As the column pressure increases, time-intensive impact of each of these characteristics indicates
the equilibrium surface concentration of adsorbed oxygen that an experimental system designed for student laboratory
rises correspondingly. In Column 2, the drop in total pres- operation should be designed for the direct study of cycle
sure reduces the partial pressure of oxygen in the gas phase, steps, rather than system performance over a number of com-
changing the system gas/surface equilibrium in the opposite plete cycles. Following this logic, a pressure swing adsorption
direction; oxygen gradually desorbs from the packing and system for the separation of air in the Unit Operations Labora-
exits the column until a new gas/surface equilibrium condi- tory was built to achieve flexible operation through hands-on
tion is reached at atmospheric pressure. manipulation and automated data acquisition.
Step 2: The product valve is opened for Column 1, and an
initially nitrogen-rich stream is sent for collection. A small LABORATORY SETTING AND
amount of this product stream is diverted to Column 2, where EXPERIMENTAL MODULE
it surrounds the packing with a low-pressure, oxygen-deficient The instructional goals and teaching methods for the Unit
environment; this causes further oxygen desorption in accor- Operations Laboratory at the Colorado School of Mines
dance with the continuing gas/surface equilibrium approach. (CSM) has been thoroughly described elsewhere.[8,9] The pri-
The vent valve from Column 2 remains open, allowing for mary functional difference between the laboratory at CSM and
the continued purging of the column. The stream diversion to its counterparts at most other universities is that it is offered
Column 2, which serves to further purge its adsorbed oxygen, as a stand-alone summer (field session) course, which allows
is known in practice as “backfilling.” In Column 1, oxygen students an extended experimental work time versus what is
from the feed air continues to adsorb on the packing at the generally possible for typical lab courses offered during an
system’s operating pressure until the packing nears saturation. academic semester or quarter. The scheduled lab times enable
46 Chemical Engineering Education
2
students to work on a given experiment for a period of up to molecules on 13X, resulting in an initially oxygen-rich gas
8 hours. Longer lab periods provide significant freedom for phase when the zeolite is exposed to air.[11] The availability of
team-by-team experimental design decisions, and addition- both packing types enables students to examine the stepwise
ally make possible the use of larger, more flexibly applied performance of a system controlled either kinetically (CMS,
laboratory modules. nitrogen production) or by surface/gas equilibrium (13X,
An operational schematic of the PSA experiment in use for oxygen production). The study of cycle steps is isolated to
the Unit Operations Laboratory at CSM is shown in Figure either nitrogen or oxygen production, so Columns A and B
2. Six process measurements are continuously logged using are not used in cycles with Columns C and D, and vice versa.
a commercial data acquisition system (DataTaker model Columns A and B contain 7.5 kg of CMS (packing density =
DT85): feed pressure (P1), purge pressure (P2), product 0.559 g cm-3, void volume = 9.35 L), and Columns C and D
temperature (T1), purge temperature (T2), input gas flow, contain 8.6 kg of 13X (packing density = 0.637 g cm-3, void
and product oxygen concentration. Students are provided volume = 9.83 L).
with a selection of feed gases for the system: compressed Exiting streams from the column may be routed to either
air, nitrogen, and a 20% oxygen mixture in argon. The an exhaust line or to a product line using another series of
compressed air is available from building utility lines three-way valves (V11 – V14). The exhaust line may either
(~ 80 psi), and the other gases are sourced from compressed vent to the atmosphere, or be connected to house vacuum (ca.
gas cylinders (regulated to ~ 20 psi). The process feed gases -12 psig) using V16. On the product line, flow is controlled
are supplied to a four-way valve (V1) with check valves to with a thermal mass flow controller (Tylan General model
prevent backflow during gas interchange. The selected feed FC-261V-4S). The exit composition is measured with an
is manually regulated, and the feed pressure is automatically electrochemical oxygen sensor (Vernier model O2-BTA),
logged with a pressure transducer (Omega model PX309- which has been provided with a higher excitation voltage (12
100GV) The feed gases are dried sequentially by refrigera- DC volts vs. the stock 5 DC volts) to increase its span from
tion (Parker model PRD15-A11516016TXU) and calcium 0 – 27% O2 to 0 – 100% O2.
sulfate desiccant packing. A bypass option is provided at
V2 to allow the feed stream to go directly to the exit line, STUDENT EXPERIMENTAL WORK AND DATA
which is a useful method for verifying the feed composition. ANALYSIS
Feed flow to the columns is measured using a thermal The instruction style for the Unit Operations Lab at CSM
mass flow meter (Aalborg model GFMS-011327). The flow requires students to thoroughly familiarize themselves with
pathways to and from the columns are controlled using the the overall system before creating a list of experimental objec-
valves on the various control panels located around the PSA tives as well as a detailed plan for achieving those objectives.
assembly. Three-way input valves (V3 – V6) allow each After a cursory analysis of the experimental system, students
column to be provided with fresh feed gas or the product of quickly realize the critical role of the oxygen sensor. Coupled
its counterpart through lines equipped with manual threaded with the feed and exit flow measurements, the oxygen sen-
flow-adjustment valves (V7 – V10). This allows for a number sor allows material balances to be carried out over time on
of experimental options—a small amount of product gas may oxygen—enabling students to determine, for example, the
be used to purge a saturated column (backfilling), pressure amount of oxygen held up in the column during an absorption
may be equalized between columns as an independent cycle trial, or the amount of oxygen released to the atmosphere dur-
step, or two columns may be connected in series when flow- ing a blowdown step after solving an oxygen balance on all
adjustment valves are fully open. other steps. Stepwise oxygen balances (and the implication of
The adsorption columns are four steel pipes (schedule 40, these balances for inferred nitrogen or argon balances) are the
10.2 cm ID), each 152 cm in length. The columns are verti- common elements of a wide range of experimental designs.
cally wall-mounted, and contain randomly packed pellets of These designs may include:
molecular sieve. The leftmost columns, designated Column A • Isolation of enriched nitrogen or enriched oxygen of a
and Column B, each contain a porous carbon molecular sieve specified purity
(CMS, Hengye CMS 260). CMS has pores with microporous • Purging of one column with product gas from another
openings[10]; these pore mouths allow gases with smaller (backfilling)
kinetic diameters (such as O2) easier access to the internal
• Application of vacuum for vacuum swing adsorption
surface than molecules with a larger kinetic diameter (such as
(VSA) study
N2). The rightmost columns, designated Column C and Col-
umn D, each contain zeolite 13X (13X, Hengye 13X812MS), • Use of columns in series or parallel arrangement
which is a silica-alumina clay with a significant amount • Pressure equalization between columns, or traditional
(up to 20%) of sodium oxide. Nitrogen molecules have a 4-step cycles
much greater surface equilibrium concentration than oxygen
Vol. 52, No. 1, Winter 2018 47
For the purposes of the present communication, the ex- 3. Blowdown: The column is returned to atmospheric
perimental goals and data analysis for one student-designed pressure as quickly as possible. When the pressure in the
experimental set are presented: the production of high-purity exhaust line reaches zero, the column exit is redirected
nitrogen from air using CMS. In this case, students chose to to the product line, and low-pressure nitrogen is intro-
duced as feed.
examine the effects of adjusting system working pressure on
the production of nitrogen from a PSA air separation cycle 4. Purge: Pure nitrogen is introduced into the column at
using CMS. The selected cycle style has four steps, and a rate of 10 L min-1 to rinse the packing of adsorbed
would in practice be very similar to the cycle shown in Figure oxygen. The oxygen sensor is monitored, and the nitro-
1(a). However, as there is no product storage vessel, purging gen feed is stopped when the exit oxygen concentration
reaches zero.
is accomplished by using nitrogen from a compressed gas
cylinder. Monitoring of effluent gas from the column in use The system outlet flow is controlled and constant, while
with the oxygen sensor is possible for every step in the cycle both the feed flow and the outlet oxygen concentration are
except blowdown, in which case the gas must be released directly measured and automatically recorded. Analysis of
very rapidly to the atmosphere from the column. The oxygen the column’s performance is typically expressed in terms of
balance for the blowdown step will therefore be determined nitrogen recovery, net product purity, adsorption analysis,
by first completing balances for the other three steps: how and cycle design through proposed step-by-step timing.
much oxygen enters the column during pressurization, how Where applicable, the oxygen concentration, feed and exit
much oxygen builds up during product collection, and how flow rates, and elapsed time for each cycle steps may be used
much is released from the surface during purging. to track the oxygen and nitrogen in the system, allowing for
An important consideration for the students involves ar- simple evaluation of product purity and product recovery.
gon (1% of the entering air) within the system. A literature For the case of nitrogen production, monitoring the oxygen
review of CMS quickly reveals the nature of its operation concentration for a known total product flow over time during
in a kinetically controlled adsorption system when applied the production step permits the direct calculation of the net
to air separation: It is expected that oxygen will be held up product purity. The calculation of nitrogen recovery requires
in the column owing to the microporous packing combined only a determination of the amount of nitrogen in the product
with oxygen’s smaller kinetic diameter versus nitrogen (3.64 as a fraction of the sum of nitrogen sent to the column (as air)
Å vs. 3.46 Å). Considering that argon has a similar kinetic during the pressurization and production steps, as well as the
diameter to oxygen gas (3.40 Å),[12] it follows that the column amount of nitrogen used during the purge step.
will effectively screen out oxygen and argon—thus, a 0% O2 Adsorption analysis is a general examination of the col-
reading at the sensor would imply a 100% N2 product stream. umn’s performance during the pressurization and produc-
The main process variable adjusted in this particular case tion steps. Generally, students find that the generation of
was the system working pressure. While house compressed air breakthrough curves can provide a number of useful system
is available at nearly 90 psig, compressor cycling and house insights; examples of these are how much capacity the adsor-
air use by other laboratory experimental stations creates a bent has under various conditions, how much of the bed may
practical upper regulated limit of about 80 psig. The students be saturated after a given time, and how all of this information
designed a series of three experiments with working pressures might apply to scaling up the PSA system to meet a specific
of 25, 50, and 75 psig. In each case, the column was purged production target.
with nitrogen gas at atmospheric pressure before beginning the The assigned text for our Unit Operations Lab[13] provides
experimental trial. Flow from the columns was always limited some general background for the analysis of adsorption in
to 10 L min-1 using the exit flow controller. The process steps packed beds. Some of the most useful material for students
were carried out as follows: performing the PSA experiment is the discussion of concen-
1. Pressurization: The air feed pressure was adjusted using tration profiles in a column during adsorption, which may
the regulator to match the selected working pressure, be inferred from breakthrough curves. An important general
and the column inlet valve was opened with the exit result relates the saturation capacity of the packing (Wsat) to
valve closed. The rate of air entering the column was the amount of time that would be required for the solute to
monitored, and when the value was equivalent to the break through in the absence of both axial dispersion and
chosen product flow rate (10 L min-1), the product valve mass transfer resistance (t*):
was opened.
u 0 c 0 t ∗ = Lρb ( Wsat − W0 ) (1)
2. Production: The composition of the gas leaving the col-
umn was monitored until its instantaneous oxygen com- Here, the superficial velocity of the fluid and its solute concen-
position reached about half of the value for air (10.5%).
tration (u0, co) are considered along with the physical proper-
The column feed was then closed, and the column exit
was redirected to the exhaust line’s atmosphere vent.
ties of the packed column: its length (L), the bulk density of
the particles within (ρb), and finally the saturation and initial
48 Chemical Engineering Education
(W 0 ) capaci-
ties of solute
on the pack-
ing. It is worth
noting that this
basic approach
to analysis of
a PSA system
focuses on ma-
terial balances
and system
performance/
scale up, so
that students
must make
simplifying
assumptions
regarding
complicated
phenomena
such as axial
dispersion and
molecular dif-
fusivity in both
the gas and
surface phases.
A more com-
plete treatment
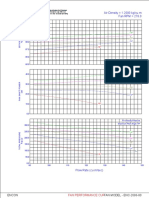
of PSA mod- Figure Figure
3. Partial3: Partial breakthrough
breakthrough curves
curves for oxygen for oxygen
adsorption adsorption
on CMS at columnon CMS pressures
working at column
(dot)
eling is found working pressures25(●) psig, (triangle) 50 psig, and (square) 75 psig.
25 psig, (▲) 50 psig, and (■) 75 psig.
elsewhere.[14]
In the case of remov- TABLE 1
al of oxygen from air Summary of column performance for the production of N2 from air using CMS packing
by adsorption on CMS, at various working pressures
the initial loading of Working Net N2 N2
t*(s)
W0 Wsat
Pressure (psig) Purity Recovery (g O2/kg CMS) (g O2/kg CMS)
oxygen on the column
packing is determined 25 98.3% 8.4% 285 2.2 4.2
using a material bal- 50 98.3% 16.9% 353 3.9 6.4
ance for the air intro- 75 98.2% 23.0% 427 5.2 8.2
duced into the column
during pressurization.
concentration of 104.5 ppt designated by the students as the
If the initial exit oxygen concentration of the product air is
production step endpoint in the experimental design.
zero, then it is reasonable for the students to assume that all
of the oxygen from the pressurization air has been adsorbed, Figure 3 shows the partial breakthrough curves obtained
determining the value for W0. The concentration of oxygen from experiments with 25, 50, and 75 psig working pressures.
in the incoming (dried) air is known (c0 = 209 ppt), and the Each trial has an identical superficial velocity of 2 cm s-1. The
superficial velocity is determined by dividing the volumetric initial amounts of oxygen adsorbed on the CMS (from pres-
flow entering the column by the nominal section area of surization) are 2.2, 3.9, and 5.2 g O2 (kg CMS)-1, respectively.
the pipe (~ 82.1 cm ). The packing length and bulk density
2 The ideal times (t*), or times to reach a concentration ratio
are provided (see the Laboratory Setting and Experimental (c c0-1) of 0.50, are, respectively, 285, 353, and 427 seconds.
Module section above). Students may therefore calculate the Table 1 summarizes the calculated outcomes for each trial.
saturation capacity (W0) of the column by measuring break- The net purity of the nitrogen product is very nearly the
through curve data up to the ideal time (t*). If a symmetric same in each case, which is a consequence of the common exit
breakthrough curve is assumed, t* will be the exit oxygen composition endpoint for each trial (c c0-1 = 0.50). However, it
Vol. 52, No. 1, Winter 2018
3 49
is clear that the gas efficiency of the process is increased automatically logged by the computerized data acquisition
as pressure is increased; more of the nitrogen that is fed to system, the manual style of operation keeps an entire working
the system is recovered as product. Although the associated group of three students actively involved in the experiment
material balances must cover pressurization, production, and without overwhelming them.
purging, students observe that the removal of waste oxygen In most chemical engineering departments, the junior- and
from the packing during blowdown is greatly reduced at senior-level laboratories are primarily regarded as the places
lower working pressures. This requires a great deal more in which the principles delivered in lecture courses are put
nitrogen use in purging, and reduces the fraction of nitrogen into practice; students get hands-on experience with process
recovered accordingly. equipment, and see things with their own eyes. At CSM, the
The experimental data may be analyzed in additional ways Unit Operations Laboratory is additionally used as a means
to provide information related to adsorber design and scale up. for driving the overall curriculum. A stand-alone, intensive
The adsorption isotherms for zeolites or other porous media summer lab course with extended laboratory working hours
used in PSA systems generally exhibit adsorption isotherms and a special emphasis on experimental design has allowed
that are classified as “favorable,” and the moving concentra- our department to make it the centerpiece of our B.S. degree
tion profile does not change shape throughout the adsorption program. As an example, the introduction of a PSA experiment
bed.[13] This allows for straightforward scaling calculations to in the laboratory spurs the inclusion of fixed-bed adsorption
be carried out based on the bed length and representative time material in the junior-level Mass Transfer (Separations) and
frames observed during breakthrough trials. The end time for senior-level Transport Phenomena courses. As a result, all
the production step in a PSA cycle, based on overall product students performing the PSA experiment for the first time have
purity design requirements, is known at the break-point time received some instruction related to the theory and practice
(tb). The ratio of the break-point time and the amount of effec- of industrial gas purification by adsorption.
tive bed length saturated at the break-point time (Lu) will be To assist in course learning outcome assessment, students
in proportion to the ideal time and the total length of the bed: are required to complete two concepts quizzes during the
tb Lu Unit Operations Laboratory—one at the course orientation,
= (2) and another on the final day of the session. To examine the
t∗ L
students’ progress in mastering the fundamental concepts
This relation is easily applied to breakthrough curve data. behind PSA systems and their design, specific questions
For example, should the students choose a break-time con- related to the experiment were created. Table 2 shows that
centration of c c0-1 = 0.10 for the 75 psig working pressure, a students in recent lab sessions have shown improvement in
net product purity of 99.9% N2 would result. The data show both theory and applied system-related questions; generally,
that this break-point concentration ratio occurs at a time of a much greater improvement is observed in the practical
318 seconds (5.3 minutes), which is 74.5% of the ideal time. aspect. Conversations with students over the course of the
Therefore, an effective 114 cm of the column length will have session indicate that an improvement of practical system
been saturated. Similarly, if a production step time of 1300 understanding is a result of the in-depth literature reviews
seconds is required for proper cycle timing, a larger column required for report introductions. Additionally, students ap-
with the same diameter must provide an ideal time of close pear to make better connections between theory and experi-
to half an hour—and through the proportionality stipulated ment after performing detailed data analysis and evaluation
by Eq. (1), it will require a length of about 6 meters. in these reports.
STUDENT EXPERIENCES AND LABORATORY Student feedback regarding the experiment itself has been
generally positive. In end-of-session course reviews, the PSA
ASSESSMENT
system is often referred to in terms such as “the experiment
The PSA system in our Unit Operations Laboratory is an I learned the most from, but which was the most difficult to
excellent example of the type of experimental system that conceptually understand.” Students also indicate an appre-
works very well in a laboratory setting in which students ciation of the provided data-acquisition system, which gives
are given a great deal of freedom in the creation of an ex- freedom to the entire team to manipulate the experiment
perimental design and also in the selection of experimental throughout the course of the day. There is always a special
objectives. Since its construction and commissioning in the focus on explaining deviations in observed data trends from
summer of 2013, the PSA system has proven to be extremely theory or pre-lab expectations. The availability of extensive
flexible in its application. automatic data sets has often provided students with additional
Students have chosen to study N2 or O2 production, overall material for analysis and explanations of such deviations.
cycle design or cycle step evaluation, the study of system scale More often than not, this leads to a deeper understanding of
up or the investigation of overall mass transfer coefficients, the physical/chemical phenomena around which PSA systems
and so on. Although most of the routine measurements are are designed.
50 Chemical Engineering Education
TABLE 2
PSA-related concept quiz questions and correct response rates from summer field sessions 2014-2016.
The total number of student responses is 343.
Pre-course Correct Post-course Correct
Concept Question Question Type
Response Rate Response Rate
(T/F) In a PSA system, it is typical for multiple layers of
Theoretical 66% 87%
adsorbed gas to form on the surface.
(T/F) Reversible gas adsorption is best achieved with the
Theoretical 71% 92%
physical adsorption of species, rather than chemisorption.
Use a sketch to show the effect of axial dispersion on a break-
Theoretical 24% 54%
through curve in a PSA system.
How may an adsorption isotherm be modeled at low solute
Theoretical 36% 60%
pressures or concentrations?
List three types of adsorbents used in gas separation pro-
Applied 15% 90%
cesses.
Briefly describe the importance of a stable column tempera-
Applied 38% 94%
ture in a PSA system.
A PSA adsorption bed requires 6 kg (10 L) of packing. Is it
better to use a column with h = 2 m and d = 8 cm, or h = 8 m Applied 29% 85%
and d = 4 cm? Why?
You are designing a PSA system, and may choose molecular
sieve stock with either a 5 mm or 0.5 mm average diameter.
Applied 77% 94%
List one advantage (or disadvantage) of each potential pack-
ing.
CONCLUSIONS Air Separation on a Carbon Molecular Sieve – II. Investigation of a
Modified Cycle With Pressure Equalization and No Purge,” Chem.
Air separation by PSA is a very effective, inherently safe, Eng. Science, 42(8), 2037 (1987)
and extremely flexible teaching and learning tool for the Unit 4. Liow, J-L., and C.N. Kenney, “The Backfill Cycle of the Pressure
Operations Laboratory. This experiment illustrates several Swing Adsorption Process,” AIChE Journal, 36(1), 53 (1990)
5. Shin, H-S., and K.S. Knaebel, “Pressure Swing Adsorption: An Ex-
interrelated principles of mass transfer, and does so as a perimental Study of Diffusion-Induced Separation,” AIChE Journal,
system that uses no harmful chemicals, produces no waste, 34(9), 1409 (1988)
and requires essentially no maintenance. Through several 6. Raghavan, N.S., M.M. Hassan, and D.M. Ruthven, “Numerical Simu-
summers of use, students have explored a wide variety of lation of a PSA System Using a Pore Diffusion Model,” Chem. Eng.
Science, 41(11), 2787 (1986)
experimental designs for both oxygen and nitrogen produc- 7. Lee, J-G., J-S. Lee, and C-H. Lee, “Air Separation by a Small-Scale
tion cycles. Written and oral reports prepared by the students Two-Bed Medical O2 Pressure Swing Adsorption,” Industrial & En-
performing the PSA experiment involve the theoretical aspects gineering Chemical Research, 40, 3647 (2001)
of solid/gas adsorption equilibrium and kinetics while plac- 8. Miller, R.L., J.F. Ely, R.M. Baldwin, and B.M. Olds, “Higher-Order
Thinking in the Unit Operations Laboratory,” Chem. Eng. Ed., 32(2),
ing appropriate focus on the real-world aspects of functional 146 (1998)
PSA system design, operation (cycle) specification, and scale 9. Ganley, J.C., “Enhanced Experiential Learning in the Unit Operations
up. Finally, questioning the students on system concepts Laboratory,” Proceedings of the ASEE Rocky Mountain Section Confer-
shows that this laboratory module is very helpful in terms ence, 114 – 123 (2016)
10. Hassan, M.M., and D.M. Ruthven, “Air Separation by Pressure Swing
of experiential learning—allowing students to build on the Adsorption on a Carbon Molecular Sieve,” Chem. Eng. Science, 41(5),
foundational knowledge delivered in earlier, lecture-based 1333 (1986)
transport courses. 11. Farooq, S., D.M. Ruthven, and H.A. Boniface, “Numerical Simulation
of a Pressure Swing Adsorption Oxygen Unit,” Chem. Eng. Science,
44(12), 2809 (1989)
REFERENCES 12. Baron, G.V., M. Van de Voorde, H. Verelst, J. Martens, and P. Jacobs,
1. Pruksathorn, P., and T. Vitidsant, “Production of Pure Ethanol from Diffusion in Zeolite Adsorbants. In M. Suzuki (Ed.), Fundamentals of
Azeotropic Solution by Pressure Swing Adsorption,” Korean J. Chemi- Adsorption, vol. 80 (pp. 49) Tokyo, Japan: Kodansha. (1993)
cal Engineering, 26(4), 1106 (2009) 13. McCabe, W.L., J.C. Smith, and P. Harriot, Unit Operations of Chemical
2. Ruthven, D.M., and S. Farooq, “Air Separation by Pressure Swing Engineering, 7th Ed. New York: McGraw-Hill (2005)
Adsorption,” Gas Separation and Purification, 4, 141 (1990) 14. Ruthven, D.M., S. Farooq, and K.S. Knaebel, Pressure Swing Adsorp-
3. Hassan, M.M., N.G. Raghavan, and D.M. Ruthven, “Pressure Swing tion, Weinheim, Germany: Wiley-VCH (1993) p
Vol. 52, No. 1, Winter 2018 51
You might also like
- A PSA Process For An Oxygen ConcentratorDocument140 pagesA PSA Process For An Oxygen ConcentratorExplorer TekiNo ratings yet
- Wind Turbine Power Calculation PDFDocument5 pagesWind Turbine Power Calculation PDFsmartman35No ratings yet
- Screw CompDocument6 pagesScrew ComplaxminarayanNo ratings yet
- Phase Equilibria: Basic Principles, Applications, Experimental TechniquesFrom EverandPhase Equilibria: Basic Principles, Applications, Experimental TechniquesNo ratings yet
- Ammonia Flooded EvaporatorDocument3 pagesAmmonia Flooded Evaporatorreclatis14100% (1)
- Analysis and Enhancement of Hydraulic Ram Pump Using Computational Fluid Dynamics (CFD)Document25 pagesAnalysis and Enhancement of Hydraulic Ram Pump Using Computational Fluid Dynamics (CFD)IJIRSTNo ratings yet
- E-03.0) General Static-Mixing Customer Specification QuestionnaireDocument6 pagesE-03.0) General Static-Mixing Customer Specification QuestionnaireWladimir Antonio Egurrola RomeroNo ratings yet
- Calculation of Flow Through Nozzles and OrificesDocument12 pagesCalculation of Flow Through Nozzles and OrificesrelaxmanNo ratings yet
- AcousticsDocument12 pagesAcousticsaguzhNo ratings yet
- Atmospheric Air EjectorsDocument2 pagesAtmospheric Air EjectorsnarupvNo ratings yet
- Sizing of Relief Valves For Two-Phase Flow in The Bayer ProcessDocument11 pagesSizing of Relief Valves For Two-Phase Flow in The Bayer Processjonyboy_1234915100% (1)
- Comp Gas Flow FormulaDocument10 pagesComp Gas Flow FormulajamestppNo ratings yet
- Incropera AnswersDocument26 pagesIncropera AnswersDhanish KumarNo ratings yet
- VALTEK Valve Size PDFDocument16 pagesVALTEK Valve Size PDFalbahbahaneeNo ratings yet
- Design Rules For Vacuum ChambersDocument12 pagesDesign Rules For Vacuum ChambersDavid Jimenez GonzalezNo ratings yet
- Sizing Technical Brief FinalDocument3 pagesSizing Technical Brief FinalBelalNorNo ratings yet
- 4 - Engine Performance ParametersDocument20 pages4 - Engine Performance ParametersWHO IS ITNo ratings yet
- Geologic Time Scale ActivityDocument10 pagesGeologic Time Scale Activityno thankyouNo ratings yet
- Gas Property+flow Eqn+ Pdrop Due To Friction ch1,2Document107 pagesGas Property+flow Eqn+ Pdrop Due To Friction ch1,2SHOBHIT KUMAR100% (1)
- Sparger Design GuideDocument5 pagesSparger Design GuideShooeibNo ratings yet
- Steam TurbinesDocument8 pagesSteam TurbinesFachri Ramadhan100% (1)
- PDF Refrigeration Compressor Driver SelectionDocument27 pagesPDF Refrigeration Compressor Driver SelectionDjamel EeddinNo ratings yet
- TurbomachineryDocument64 pagesTurbomachineryStefan Arichta100% (1)
- Advances in PSADocument14 pagesAdvances in PSAmichaelpardoNo ratings yet
- Bolt Joint-DiagramsDocument6 pagesBolt Joint-Diagramskulov1592No ratings yet
- Gas Flow in PipelineDocument18 pagesGas Flow in Pipelineamaleena_muniraNo ratings yet
- How To Select Turbomachinery For Your ApplicationDocument10 pagesHow To Select Turbomachinery For Your ApplicationSubhash PadmanabhanNo ratings yet
- An Introduction To Boiler Design Basics: Budi L HakimDocument99 pagesAn Introduction To Boiler Design Basics: Budi L HakimJaya DiNo ratings yet
- Fluid Mechanics and Heat Transfer With No Newtonian Liquids in Mechanically Agitated VesselsDocument102 pagesFluid Mechanics and Heat Transfer With No Newtonian Liquids in Mechanically Agitated VesselsgpcshfNo ratings yet
- Sulfur Tank Heat LossDocument17 pagesSulfur Tank Heat LossAnonymous oVRvsdWzfBNo ratings yet
- Glycol Water MixtureDocument4 pagesGlycol Water MixtureMohsin MohammedNo ratings yet
- Comparison of Cryogenic Flow Boiling in Liquid Nitrogen and Liquid Hydrogen Chilldown ExperimentsDocument12 pagesComparison of Cryogenic Flow Boiling in Liquid Nitrogen and Liquid Hydrogen Chilldown Experimentsnewrajasingh100% (1)
- Numerical Simulation of Multiphase Reactors with Continuous Liquid PhaseFrom EverandNumerical Simulation of Multiphase Reactors with Continuous Liquid PhaseNo ratings yet
- Automotive Radiator - Full-LibreDocument10 pagesAutomotive Radiator - Full-LibreLalitVohraNo ratings yet
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationFrom EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationNo ratings yet
- Vacuum Engineering Calculations, Formulas, and Solved ExercisesFrom EverandVacuum Engineering Calculations, Formulas, and Solved ExercisesRating: 4.5 out of 5 stars4.5/5 (2)
- Handbook of Thermal Conductivity, Volume 2: Organic Compounds C5 to C7From EverandHandbook of Thermal Conductivity, Volume 2: Organic Compounds C5 to C7No ratings yet
- The Low-Carbon Steam Plant Room BrochureDocument8 pagesThe Low-Carbon Steam Plant Room BrochureFernando CeballosNo ratings yet
- Giraldo 2019 Political - Ecology - of - AgricultureDocument161 pagesGiraldo 2019 Political - Ecology - of - AgriculturecatalinaNo ratings yet
- Handbook of Thermal Conductivity, Volume 1: Organic Compounds C1 to C4From EverandHandbook of Thermal Conductivity, Volume 1: Organic Compounds C1 to C4Rating: 5 out of 5 stars5/5 (1)
- U Control For Cryogenic Turboexpanders PDFDocument6 pagesU Control For Cryogenic Turboexpanders PDFJose Luis Rodriguez LópezNo ratings yet
- Compressor Stage Pressure - Design & OptimizationDocument4 pagesCompressor Stage Pressure - Design & OptimizationAshwin ChandaranaNo ratings yet
- Thermodynamics An Engineering Approach 8Document1 pageThermodynamics An Engineering Approach 8Aracely PenaNo ratings yet
- Anti-Surge - Valve - ROI - REXA - 2Document10 pagesAnti-Surge - Valve - ROI - REXA - 2Hash LalaNo ratings yet
- The Optimal Design of Pressure Swing Adsorption SystemsDocument27 pagesThe Optimal Design of Pressure Swing Adsorption SystemsBich Lien PhamNo ratings yet
- Screw Compressors ReviewDocument19 pagesScrew Compressors ReviewCarlos Maldonado AlmeidaNo ratings yet
- Ethylene GlycolDocument8 pagesEthylene GlycoljeswinNo ratings yet
- Air CoolerDocument15 pagesAir Coolerronny_fernandes363No ratings yet
- Thermo Coupled Stress Analysis of Exhaust Manifold Assemblage Using ABAQUSDocument6 pagesThermo Coupled Stress Analysis of Exhaust Manifold Assemblage Using ABAQUSInfogain publicationNo ratings yet
- Is 3224 2002Document47 pagesIs 3224 2002suresh kumar100% (1)
- SIMONE (Equations and Methods)Document65 pagesSIMONE (Equations and Methods)Amine DoumiNo ratings yet
- Design, Construction and Testing of An Outward Radial-Flow Reaction Water TurbineDocument97 pagesDesign, Construction and Testing of An Outward Radial-Flow Reaction Water TurbineMos Craciun100% (1)
- Valve ModelingDocument10 pagesValve ModelingmsNo ratings yet
- ASME Code and Rupture Discs Requirements-FikeDocument4 pagesASME Code and Rupture Discs Requirements-FikeMaha Wasif Khan100% (1)
- Steam Air Ejector Performance and Its Dimensional ParametersDocument296 pagesSteam Air Ejector Performance and Its Dimensional ParametersGuru Raja Ragavendran NagarajanNo ratings yet
- About Jockey PumpsDocument4 pagesAbout Jockey PumpsChemical.AliNo ratings yet
- Gas Hydrate Tutorial PDFDocument134 pagesGas Hydrate Tutorial PDFGinoNo ratings yet
- sd7100 - SYLOBEAD® MS 514NGD (MADE IN EU) - (GB)Document13 pagessd7100 - SYLOBEAD® MS 514NGD (MADE IN EU) - (GB)dilip matalNo ratings yet
- sd7100 - SYLOBEAD® MS 512 (MADE IN EU) - (IN1)Document9 pagessd7100 - SYLOBEAD® MS 512 (MADE IN EU) - (IN1)dilip matalNo ratings yet
- Permitted - Securities - Xls (1) - 0Document1 pagePermitted - Securities - Xls (1) - 0dilip matalNo ratings yet
- MEV11224163 ODUM - Technical OfferDocument7 pagesMEV11224163 ODUM - Technical Offerdilip matalNo ratings yet
- DCS GraphicsDocument5 pagesDCS Graphicsdilip matalNo ratings yet
- 89 Gas and Oilfield Safety Inspection ChecklistDocument14 pages89 Gas and Oilfield Safety Inspection Checklistdilip matalNo ratings yet
- Astm TablesDocument253 pagesAstm Tablesdilip matalNo ratings yet
- CAL Gas CompositionDocument1 pageCAL Gas Compositiondilip matalNo ratings yet
- sd8616 - CERAMIC BALLS (MADE IN EU) - (GB)Document8 pagessd8616 - CERAMIC BALLS (MADE IN EU) - (GB)dilip matalNo ratings yet
- DC4 Data SheetDocument2 pagesDC4 Data Sheetdilip matalNo ratings yet
- Restriction Orifice-FKDocument2 pagesRestriction Orifice-FKdilip matalNo ratings yet
- Performance Curves - 13 FeetDocument1 pagePerformance Curves - 13 Feetdilip matalNo ratings yet
- Water Fire Truck-XCMG-7.10.2019Document6 pagesWater Fire Truck-XCMG-7.10.2019dilip matalNo ratings yet
- A 371Document1 pageA 371dilip matalNo ratings yet
- PHA-Template-Chemical Feeding SystemDocument8 pagesPHA-Template-Chemical Feeding Systemdilip matalNo ratings yet
- 7' Diameter Fan Ga DrawingDocument1 page7' Diameter Fan Ga Drawingdilip matalNo ratings yet
- HAZOP Technical InputsDocument6 pagesHAZOP Technical Inputsdilip matalNo ratings yet
- GC Span Gas CompositionDocument1 pageGC Span Gas Compositiondilip matalNo ratings yet
- Dryer Performance MonitoringDocument3 pagesDryer Performance Monitoringdilip matalNo ratings yet
- Quotation For AGILENT CHROMATOGRAPHY INSTALLATIONDocument2 pagesQuotation For AGILENT CHROMATOGRAPHY INSTALLATIONdilip matalNo ratings yet
- Ahl Condensate-@mainDocument26 pagesAhl Condensate-@maindilip matalNo ratings yet
- Scheduled of Lab Routine AnalysisDocument3 pagesScheduled of Lab Routine Analysisdilip matalNo ratings yet
- CalculationDocument2 pagesCalculationdilip matalNo ratings yet
- Instrument Data For Coriolis Flow TransmitterDocument2 pagesInstrument Data For Coriolis Flow Transmitterdilip matalNo ratings yet
- Acid Gas Cleaning Caustic Wash Model SummaryDocument5 pagesAcid Gas Cleaning Caustic Wash Model Summarydilip matalNo ratings yet
- TECHNICAL DATASHEET FOR - For Supply of Amine - Plate & Frame ExchangerDocument4 pagesTECHNICAL DATASHEET FOR - For Supply of Amine - Plate & Frame Exchangerdilip matalNo ratings yet
- Community Issues DATEDocument1 pageCommunity Issues DATEdilip matalNo ratings yet
- Slug Catcher Multiple Pipe Sizing Finger TypeDocument7 pagesSlug Catcher Multiple Pipe Sizing Finger Typedilip matalNo ratings yet
- Condensate With CompDocument14 pagesCondensate With Compdilip matalNo ratings yet
- Report 20230109Document4 pagesReport 20230109dilip matalNo ratings yet
- Group-4 Assignment 3.2 Technology As Way of Revealing (Midterm Project)Document11 pagesGroup-4 Assignment 3.2 Technology As Way of Revealing (Midterm Project)JOHN DAVE RAMIREZNo ratings yet
- Geothermal Energy: Utilization As A Heat Pump: Mr. A. M. Vibhute, Prof. S.M.Shaikh, Prof. A. M. PatilDocument5 pagesGeothermal Energy: Utilization As A Heat Pump: Mr. A. M. Vibhute, Prof. S.M.Shaikh, Prof. A. M. PatilSongül BayındırNo ratings yet
- DRRR InfographicDocument2 pagesDRRR Infographiccarle100% (1)
- Rachmawati 2021 IOP Conf. Ser. Mater. Sci. Eng. 1115 012068 PDFDocument9 pagesRachmawati 2021 IOP Conf. Ser. Mater. Sci. Eng. 1115 012068 PDFKristel ComitanNo ratings yet
- EVS Semester II Module I Environmental Governance and ManagementDocument35 pagesEVS Semester II Module I Environmental Governance and ManagementKhushbooNo ratings yet
- Borosil RenewablesDocument2 pagesBorosil RenewablesJatin SoniNo ratings yet
- El FuegoDocument5 pagesEl FuegojitgeNo ratings yet
- 50 Mechanical Engineering Interview Questions and AnswersDocument12 pages50 Mechanical Engineering Interview Questions and Answersmj03127477706No ratings yet
- AssfDocument8 pagesAssfWinni TanNo ratings yet
- IES Data SheetDocument2 pagesIES Data SheetBreathing BuildingsNo ratings yet
- Lecture 11 - Forced ConvectionDocument35 pagesLecture 11 - Forced ConvectionNiaz Muhammad Khan MehmotiNo ratings yet
- Questions CombustionDocument3 pagesQuestions CombustionKristian TarucNo ratings yet
- Question 966541Document4 pagesQuestion 966541gauravgambhirsonowalNo ratings yet
- The Hydrological System PDFDocument30 pagesThe Hydrological System PDFEngr.Hamid Ismail CheemaNo ratings yet
- Flooded Return Targeted Return Contained Return: Small LAN Rooms 40kW General Use Large Data Center / ColocationDocument3 pagesFlooded Return Targeted Return Contained Return: Small LAN Rooms 40kW General Use Large Data Center / Colocationfernanda boldtNo ratings yet
- Archimedes principle equation: Mass Density × V olume Ρ × VDocument2 pagesArchimedes principle equation: Mass Density × V olume Ρ × VAbdullah Ahsan AhmedNo ratings yet
- Page of Paper Trick-4-ScienceDocument2 pagesPage of Paper Trick-4-ScienceKirti SharmaNo ratings yet
- A - Vapour Compression Test RigDocument36 pagesA - Vapour Compression Test RigProf. Jitendra SatputeNo ratings yet
- US Declaration of Interdependence 1976Document10 pagesUS Declaration of Interdependence 1976FranSwizzle SimsNo ratings yet
- 06-Shubham-A Study of Green Accounting Practices in IndiaDocument6 pages06-Shubham-A Study of Green Accounting Practices in IndiaRahul AroraNo ratings yet
- Elements of Environmental Studies: November 2018Document13 pagesElements of Environmental Studies: November 2018Sponge BobNo ratings yet
- 04 - AbsorbersDocument11 pages04 - AbsorbersRafael ReyesNo ratings yet
- Tutorial 2Document3 pagesTutorial 2GabrielNo ratings yet