Professional Documents
Culture Documents
Section A Multiple Choice Questions (20 Marks)
Uploaded by
Timothy HandokoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Section A Multiple Choice Questions (20 Marks)
Uploaded by
Timothy HandokoCopyright:
Available Formats
2
Section A Multiple Choice Questions (20 marks)
For Questions 1 to 15, four possible answers labelled A, B, C and D are given for
each question.
Choose the one you consider to be correct and indicate your answers on the Answer
booklet provided.
1 U3O8 is a catalyst, prepared by heating a uranium nitrate salt, UO2(NO3)2. The
salt decomposes quantitatively to give U3O8, NO2 and O2 only. How many
moles of O2 are produced from one mole of the salt?
A 1/3
B 1/2
C 2/3
D 2
2 The main ingredient in chocolate bars is sugars, typically about 47% in milk
chocolate bars. If these sugars are represented by sucrose, C12H22O11, how
many sugar molecules are there in 1 kg of chocolate bars?
A 9.67 x 1022
B 2.82 x 1023
C 8.27 x 1023
D 3.57 x 1024
3 A constituent of wood preservative is manufactured by heating the ore chromite,
FeCr2O4, with sodium carbonate in air.
4FeCr2O4 + 8Na2CO3 + 7O2 8Na2CrO4 + 2Fe2O3 + 8CO2
What are the changes in oxidation number of chromium and iron in this
reaction?
Change in oxidation number Change in oxidation number
of chromium of iron
A +1 0
B +2 0
C +2 +1
D +3 +1
© ACJC 2014 JC1 Term Exam 2014
3
4 Use of the Data Booklet is relevant to this question.
Potassium manganate(VII), KMnO4, reacts with sodium cyanide, NaCN, in a
molar ratio of 2:3 to form manganese(IV) oxide, MnO2.
Which of the following would be the carbon-containing product in this reaction?
A* C
B D
5 In which of the following compounds does the underlined element contain
unpaired electrons?
A PH3
B MnCl2
C SO3
D Cu2O
6 The table below contains incomplete information of two ions, Y and Z.
Ion Mass Atomic Number of Number of Charge of
Number Number neutrons electrons ion
Y 59 27 e f +2
Z 79 g 45 36 h
Which of the following shows the missing numbers (e, f, g and h) in the above
table?
e f g h
A 29 27 34 +2
B 32 27 34 -2
C 59 25 36 +2
D 32 25 34 -2
© ACJC 2014 JC1 Term Exam 2014
4
7 Under what conditions will the behaviour of a gas be least ideal?
pressure temperature
A high high
B high low
C low low
D low high
8 Two identical bulbs at the same temperature contain ideal gases D and E
separately. The density of gas D is twice that of gas E and the molecular mass
of gas D is half that of gas E.
What is the ratio of the pressure of gas D to that of gas E?
A 1:2 B 1:1 C 2:1 D 4:1
Which bonding does not correspond to its description of physical properties?
9
Bonding type Physical properties
A Ionic High melting point, conducts electricity when
molten but not when solid
B Metallic Variety of melting points, conducts electricity
when solid and when molten
C Giant covalent High melting point, conducts electricity
in the aqueous state but not when solid
D Simple covalent Low melting point, does not conduct
electricity in solid, liquid and gaseous states
10 Why does copper wire conduct electricity when a potential difference is
applied?
A The crystal lattice breaks down.
B The atoms of copper become ionised.
C Copper ions move in the crystal lattice.
D Bonding electrons in the crystal lattice move.
© ACJC 2014 JC1 Term Exam 2014
5
11 Which isomer of the hydrocarbon C7H16 is likely to have the highest boiling
point?
A (CH3)3CCH(CH3)2
B (CH3)2CHCH2CH(CH3)2
C CH3CH2CH(CH3)CH(CH3)2
D CH3CH2CH2CH2CH2CH2CH3
12 Boron nitride, BN, exists in two possible forms, hexagonal boron nitride and
cubic boron nitride. The structure of hexagonal boron nitride and cubic boron
nitride are similar to graphite and diamond respectively.
boron atom
nitrogen atom
hexagonal boron nitride cubic boron nitride
Which of the following statements is incorrect?
A They are both giant covalent structures.
B There is dative bonding in cubic boron nitride.
C The layers in hexagonal boron nitride are held together by van der
Waals’ forces.
D Only weak van der Waals’ forces are overcome when melting
hexagonal boron nitride.
13 Which of the following pairs does species I have a smaller bond angle than
species II?
I II
A NO3– NO2–
B SF6 ClF3
C XeF4 BrF5
D BrO3– ClO3–
© ACJC 2014 JC1 Term Exam 2014
6
14 The diagram represents the melting points of four consecutive elements in the
third period of the Periodic Table.
melting point / K
0 proton number
The sketches below represent another two properties of the elements.
property M property N
0 proton number 0 proton number
What are properties M and N?
property M property N
A third ionisation energy electronegativity
B number of valence electrons boiling point
C ionic radius effective nuclear charge
D electrical conductivity atomic radius
© ACJC 2014 JC1 Term Exam 2014
7
15 A student used the set-up below to heat a can containing 200 g of water.
thermometer
can containing
200 g of water
burner containing
propan-1-ol
You are given the following:
Change in temperature of water = ΔT oC
Relative molecular mass of propan-1-ol = 60.0
Enthalpy change of combustion of propan-1-ol = 2021 kJ mol−1
Specific heat capacity of water = c J g−1 K−1
Assuming that efficiency of this process is 80%, what is the mass of propan-1-ol
burnt?
A 2021 1000 0.8 C 200 c ΔT 60.0 0.8
200 c ΔT 60.0 2021
B 2021 1000 D* 200 c ΔT 60.0
c ΔT 0.8 2021 1000 0.8
From questions 16 to 20, one or more of the three numbered statements 1 to 3 may be correct.
Decide whether each of the statements is or is not correct (you may find it helpful to put a tick
against the statements that you consider to be correct).
The responses A to D should be selected on the basis of
A B C D
1, 2 and 3 1 and 2 2 and 3 1 only
are only are only are is
correct correct correct correct
No other combination of statements is used as a correct response.
16 Which of these statements correctly describes atomic orbitals?
1 The energy of a d orbital is always higher than that of an s orbital
for any given quantum shell.
2 Electrons in a 3p orbital are more diffused than those in a 2p
orbital.
3 For elements in the ground state, electrons always fill up a
spherical orbital before a dumbbell shaped orbital of the same
principal quantum number.
© ACJC 2014 JC1 Term Exam 2014
8
The responses A to D should be selected on the basis of
A B C D
1, 2 and 3 1 and 2 2 and 3 1 only
are only are only are is
correct correct correct correct
No other combination of statements is used as a correct response.
17 For a fixed mass of an ideal gas, which of the following graphs do/does not
have the same general shape as the graph of P against ρ T?
(ρ = density of the gas; M = molar mass of gas)
1* M
PV against
T
2 P
against T
3 T
against V
P
18 An element X forms an ion Xn-.
Which of the following statements about X and Xn- are correct?
1 The radius of Xn- is smaller than that of X.
2 Less energy is released when an electron is added to Xn- than to X.
Less energy is required to remove the outermost electrons from Xn-
3
than from X.
19 Which of the following exhibit hydrogen bonding?
1 CH3F
2 KHF2
3 CH3CH2OH
θ
20 Which of the following reactions does the value of H represent both standard
enthalpy change of combustion and standard enthalpy change of formation?
1 P4(s) + 5O2(g) P4O10(s)
2 ½N2(g) + ½O2(g) NO(g)
3 2Mg(s) + O2(g) 2MgO(s)
END OF SECTION A
© ACJC 2014 JC1 Term Exam 2014
9
Section C (15 marks)
Answer all questions on foolscap paper.
1 Boron and aluminium are elements from Group III, where both form
trihalides with chlorine and fluorine.
(a) Boron is a poor conductor of electricity at room temperature and has an [2]
unusually high melting point of 2076 oC whereas aluminium has a melting
point of 660 oC.
Suggest the structure and bonding of boron.
(b) (i) Draw the dot-and-cross diagram for boron trifluoride and state its shape [3]
and bond angle.
(ii) In an attempt to determine the bond energies of B-F and B-Cl [1]
theoretically, a student found the following data.
Standard enthalpy change of formation of BF3 (g) / kJ mol-1 -1039
Standard enthalpy change of formation of BCl 3 (g) / kJ mol-1 -439
Standard enthalpy change of atomisation of B (s) / kJ mol-1 563
From the value above, define standard enthalpy change of formation of
boron trifluoride.
(iii) Using the data from the Data Booklet and the above table, determine the [4]
bond energies of B-F and B-Cl by constructing a general energy cycle.
You may use X2 to represent the halogens at standard state.
(iv) Hence, explain the difference in bond energy for B-F and B-Cl. [1]
(c) Aluminium chloride exists as a dimer whereas aluminium fluoride and
boron trichloride do not.
(i) Draw the structure of the dimer of aluminium chloride and explain how it [2]
is formed.
(ii) Suggest why alumiumium fluoride and boron trichloride do not exist as [2]
dimers.
END OF SECTION C
© ACJC 2014 JC1 Term Exam 2014
10
Section C (15 marks)
1 Boron and aluminium are elements from Group III, where both form
trihalides with chlorine and fluorine.
(a) Boron is a poor conductor of electricity at room temperature and has [2]
an unusually high melting point of 2076 oC whereas aluminium has a
melting point of 660 oC.
Suggest the structure and bonding of boron.
Giant covalent structure [1] with strong covalent bonds between
boron atoms [1]
(b) (i) Draw the dot-and-cross diagram for boron trifluoride and state its [3]
shape and bond angle.
xx
xx
xx
F
x
B
xx x x xx
xx
xx
F F
xx xx
[1]
Trigonal planar [1]
Bond angle: 120o [1]
(ii) In an attempt to determine the bond energies of B-F and B-Cl [1]
theoretically, a student found the following data.
Standard enthalpy change of formation of BF3 (g) / kJ mol-1 -1039
Standard enthalpy change of formation of BCl 3 (g) / kJ mol-1 -439
Standard enthalpy change of atomisation of B (s) / kJ mol-1 563
From the value above, define enthalpy change of formation of boron
trifluoride.
Enthalpy change of formation of boron trifluoride is the amount
of energy given out when 1 mol of boron trifluoride is formed
from boron and fluorine at the standard state, at 1 atm and 298K.
(iii) Using the data from the Data Booklet and the above table, determine [4]
the bond energies of B-F and B-Cl by constructing a general energy
cycle. You may use X2 to represent the halogens at standard state.
∆Hf θ(BX3)
B (s) + 3/2 X2 (g) BX3 (g)
563 + 3/2BDE(X-X) 3BDE(B-X)
B (g) + 3X (g)
[1] – correct state symbols and balanced equation
[1] – Correct direction of arrows + correct label
By Hess’ Law,
BDE(B-X) = [563 + 3/2BDE(X-X) - ∆Hf θ(BX3)]/3
BDE(B-F) = + 613 kJmol-1 [1]
BDE(B-Cl) = + 456 kJmol-1 [1]
© ACJC 2014 JC1 Term Exam 2014
11
(iv) Hence, explain the difference in bond energy for B-F and B-Cl. [1]
B-F bond is stronger than B-Cl due to smaller size of F atom and
therefore, more effective orbital overlap between B and F than
that between B and Cl.
(c) Aluminium chloride exists as a dimer whereas aluminium fluoride and
boron trichloride do not.
(i) Draw the structure of the dimer of aluminium chloride and explain [2]
how it is formed.
Cl Cl Cl
Al Al
Cl Cl Cl
[1]
Al is electron deficient with an empty orbital which will accept a
lone pair of electrons from Cl. [1]
(ii) Suggest why alumiumium fluoride and boron trichloride do not exist [2]
as dimers.
Aluminium fluoride has strong electrostatic attraction between
aluminium cations and fluoride anions in a giant ionic lattice
structure and therefore does not exist as simple covalent
dimers. [1] OR Aluminium fluoride is an ionic compound with
giant ionic lattice structure.
Boron trichloride is unable to form dimers due to the small size
of boron atom, preventing it from forming bonds with 4 other
larger Cl atoms.
OR
There will be repulsion between the larger Cl atoms that
prevents dative bond from forming between B and Cl. [1]
© ACJC 2014 JC1 Term Exam 2014
2
Section A (20 marks)
Write your answers to the multiple choice questions in the table provided
below.
1. C 6. D 11. D 16. A
2. C 7. B 12. D 17. D
3. D 8. D 13. D 18. C
4. A 9. C 14. A 19. C
5. B 10. D 15. D 20. D
Section B (25 marks)
Answer all questions in the spaces provided on the question paper.
1 Ethanedioic acid has the formula (COOH)2 and is a dibasic acid which is
readily oxidised to carbon dioxide by hot acidified potassium manganate
(VII).
(a) Construct the overall balanced ionic equation for the reaction between
hot acidified manganate (VII) ions and ethanedioic acid.
(COOH)2 2CO2 + 2 H+ + 2e
Overall: 5(COOH)2 + 2MnO4- + 6 H+ 2Mn2+ + 10 CO2 + 8 H2O
[1]
(b) In a practical assessment, a student was asked to determine the
concentrations of two solutions, ethanedioic acid and potassium
manganate (VII), given solid anhydrous potassium carbonate. This is
what the student plans to do:
1. Prepare a standard solution of potassium carbonate of 0.1 mol
dm-3 using solid anhydrous potassium carbonate.
2. Add different volumes of potassium carbonate to identical
samples of 25.0 cm3 ethanedioic acid solution so that partial
neutralisation occurs.
3. Warm the mixture to about 70C and titrate the remaining
ethanedioic acid in each mixture with potassium manganate (VII).
4. Plot a graph of volume of potassium manganate (VII) used (y-
axis) against volume of potassium carbonate added (x-axis) and
obtain the values of the intercepts.
5. Using the values of the intercepts, determine the concentrations
of ethanedioic acid and potassium manganate (VII).
© ACJC 2014 JC1 Term Exam 2014
3
(i) Outline the procedure involved in preparing 250 cm3 of standard
solution of 0.1 mol dm-3 of potassium carbonate using solid
anhydrous potassium carbonate.
Amt of K2CO3 to be in the standard solution = 0.1 x 0.250 = 0.025
mol
Mass of K2CO3 required = 0.025 x (39.1x2+12.0+16.0x3) = 3.46 g
[½]
1. Weigh out about 3.46 g of the anhydrous potassium
carbonate in a weighing bottle using a weighing balance.
[½]
2. Dissolve the anhydrous potassium carbonate in about
100 cm3 deionised water in a 250 cm3 beaker. [ ½ ]
3. Using a filter funnel, transfer the solution into a 250 cm3
graduated flask.
4. Rinse the beaker, glass rod and filter funnel with
deionised water and add washings into the graduated
flask. [ ½ ]
5. Make up to 250 cm3 mark using deionised water. [ ½ ]
6. Stopper and shake well to get a homogenous solution.
[3]
[½]
(ii) The student plotted the graph and obtained the following values for
the x- and y-intercepts.
x - intercept = 25.20 cm3
y - intercept = 28.10 cm3
Given that the potassium carbonate solution prepared has an actual
concentration of w mol dm-3, use the intercept value(s) to determine
the concentrations of ethanedioc acid and potassium manganate
(VII), in terms of w.
Amount of K2CO3 used to react with 25.0cm3 of ethanedioic acid
= 0.0252 w
Amount of (COOH)2 = 0.0252 w
Concentration of (COOH)2 = 0.0252x/0.025 = 1.01 w mol dm-3 [1]
Amount of KMnO4 used to react with 25.0cm3 of ethanedioic
acid = 2(0.0252 w)/5 = 0.01008 w
Concentration of KMnO4 = 0.01008 w /0.0281 = 0.359 w mol dm-3
[1]
(allow ecf for concentration of KMnO4 if amount of (COOH)2 was
calculated wrongly) [2]
[Total: 6 marks]
© ACJC 2014 JC1 Term Exam 2014
4
2 Cyanogen, a highly toxic gas, is composed of 46.2 % carbon and 53.8 %
nitrogen by mass. At 25 C and 101 kPa, 1.05 g of cyanogen occupies 0.500
dm3. By calculating the relative molecular mass of cyanogen, determine the
molecular formula. Hence, draw the dot-and-cross diagram.
C N
% by mass 46.2 53.8
No. of moles of atoms 46.2/12 = 3.85 53.8/14 = 3.84
Simplest mole ratio 1 1
Empirical formula of cyanogen is CN
1
Using PV = nRT,
1.01 x 105 x 0.500 x 10-3 = 1.05 x 8.31 x 298
Mr
Mr of cyanogen = 52.0
(CN)n = 52 1
n = 52 ÷ (12 + 14)
n = 2
Molecular formula of cyanogen is C2N2
xxx x
:N … C x C xxx
… N:
1
[3]
[Total: 3 marks]
3 (a) Write an equation to define the second ionisation energy of an element
X.
X+ (g) X2+ (g) + e-
No state symbols - 0 [1]
© ACJC 2014 JC1 Term Exam 2014
5
(b) The second ionisation energies of the elements magnesium to chlorine
are plotted in the following graph.
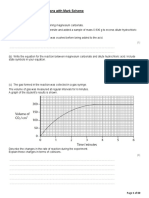
2nd Ionisation Energy of Mg to Cl
2500
2nd Ionisation Energy / kJmol-1
2260 2300
2000
1820 1580 1900
1500
1450
1000
500
0
Mg Al Si P S Cl
Element
(i) Explain the relative values of the second ionisation energies of
aluminium and silicon.
2nd IE of Al:
Al+(g) Al2+(g) + e
1s2 2s2 2p6 3s2 1s2 2s2 2p6 3s1
2nd IE of Si:
Si+(g) Si2+(g) + e
1s2 2s2 2p6 3s2 3p1 1s2 2s2 2p6 3s2
The 2nd ionisation energy of Al involves the removal of a 3s
electron or show electronic configuration of Al+
The 2nd ionisation energy of Si involves the removal of a 3p
electron or show electronic configuration of Si+ [1]
More energy is required to remove the 3s electron which
possess lower energy or closer to the nucleus [1] hence more
strongly attracted by the nucleus compared to that of the 3p [2]
electron.
© ACJC 2014 JC1 Term Exam 2014
6
(ii) How would you expect the first ionisation energy of Selenium (Se)
to compare with that of Bromine (Br)? State your reasoning.
Nuclear charge (or no. proton) of Br > Se and approximately
constant screening effect [1]
Effective nuclear charge of Br > Se
OR
Stronger electrostatic attraction between nucleus and the valence
electrons of Br
More amount of energy required to remove the valence electron of
Br
OR
1st ionisation energy of Se < Br [1] [2]
(c) The first eight ionisation energies (in kJ mol-1) of an element, T are as
follows:
966 (1st), 1950, 2730, 4850, 6020, 12130, 14330, 17830
(i) State, giving reasons, the group of the Periodic Table to which T is
likely to belong.
966 1950 2730 4850 6020 12130 14330 17830
984 780 2120 1170 6110 2200 3500
There is a big increase in the I.E. upon the removal of the 6th electron
OR
the difference between the 5th and 6th I.E. is the largest.
Hence, the first five electrons are in the outermost electron shell [1] while
the 6th electron is from the next inner electron shell.
OR
the 6th electron is removed from an inner principal quantum shell which is
nearer to the nucleus.
It can then be deduced that the element is in Group V. [1] [2]
(ii) Explain why the successive ionisation energies for element T
increase as electrons are removed successively.
Ionisation energy involves removal of electron.
The nuclear charge (or no of protons) remains the
same while the number of remaining electrons
decreases. [1] or p:e ratio increases.
Hence valence electrons are held more tightly by the
positive nucleus [1] and more energy is required to
remove the next electron. Or more energy is required to
remove an electron from an increasingly more positive ion.
The successive ionisation energy hence increases. [2]
[Total: 9 marks]
© ACJC 2014 JC1 Term Exam 2014
7
4 Some physical properties of the oxides of elements of the third period are listed
below:
Formula of Na2O MgO Al2O3 SiO2 P4O10 SO3 Cl2O
oxide
Melting point 1132 2830 2054 1710 422 17 -121
/C
Electrical
good very nil
conductivity
by molten or poor
liquid oxide
(a) With reference to structure and bonding, explain the following:
(i) P4O10 has a much lower melting point than Al2O3 and SiO2.
P4O10 has a simple molecular structure whereas Al2O3 and SiO2
have a giant ionic lattice structure and giant covalent structure
respectively.
Less energy is required to overcome the weak instantaneous dipole
– induced dipole interactions (or Van der Waals forces) between
P4O10 molecules, than in Al2O3, strong ionic bonds are broken, and
in SiO2, strong covalent bonds are broken.
Structure & bonding 1 mark for each oxide [3]
(ii) P4O10 has a higher melting point than SO3.
SO3 has a simple molecular structure.
P4O10 has more electrons, and hence, a larger electron cloud than
SO3 which is more polarisable [1]. Thus, more energy (i.e. a higher
temperature) is required to break the stronger id-id interactions
between P4O10 molecules [1]. [2]
© ACJC 2014 JC1 Term Exam 2014
8
(b) On the axes below, sketch a graph showing how electrical conductivity
varies for the oxides in their molten state.
Electrical Conductivity
Na2O MgO Al2O3 SiO2 P4O10 SO3 Cl2O
1 mark for increasing trend for Na2O, MgO & Al2O3
1 mark for SiO2 lower than the above 3 oxides & the rest as nil.
[2]
[Total: 7 marks]
END OF SECTION B
© ACJC 2014 JC1 Term Exam 2014
1
JC1 H2 Chemistry Term Exam 2015 (Suggested answers + examiners’
comments)
Section A
1
Use of the Data Booklet is relevant to this question.
In a typical solid fertiliser for use with household plants and shrubs, the
percentage by mass of the element N is 15 %. The recommended usage of this
fertiliser is 14 g per 5 dm3 of water.
What is the recommended concentration of nitrogen atoms in this solution?
A 0.03 mol dm−3
B 0.05 mol dm−3
C 0.42 mol dm−3
D 0.75 mol dm−3
Answer: A
14
Concentration of fertiliser =
5
= 2.8 g dm−3
Concentration of nitrogen in g dm−3 = 2.8 x 0.15
= 0.42 g dm−3
0.42
Concentration of nitrogen in mol dm−3 =
14
= 0.03 mol dm−3
2
0.010 moles of a hydrocarbon was burnt in excess oxygen. The resulting
gaseous mixture was passed through a first U-tube containing anhydrous CaSO4
and then through a second U-tube containing solid NaOH.
There was an increase in mass of each U-tube and these are recorded below.
Increase in mass of U-tube containing anhydrous CaSO4 / g 0.72
Increase in mass of U-tube containing solid NaOH / g 1.32
What is the relative molecular mass of the hydrocarbon?
A 40.0
B 44.0
C 60.0
D 204.0
Answer: B
Anhydrous CaSO4 absorbs water vapour so the increase in mass of U-tube
© ACJC 2015 JC1 Term Exam 2015
2
containing anhydrous CaSO4 is attributed to the mass of water.
NaOH reacts with CO2 to form Na2CO3 so the increase in mass of U-tube
containing NaOH is attributed to the mass of CO2.
0.72
Amount of H2O = = 0.04 mol
18
1.32
Amount of CO2 = = 0.03 mol
44
Amount of hydrocarbon = 0.01 mol
CxHy ≡ 4H2O ≡ 3CO2
So x = 3, y = 8.
Mr (C3H8) = 44.0
3 0.010 moles of metal, M, reacted with 0.015 moles of chlorine gas to form only
one product. Determine the empirical formula of this product.
A M3Cl2
B MCl3
C M2Cl3
D MCl2
Answer: B
Amount of M : Amount of Cl2
2 : 3
2M + 3Cl2 → M2Cl6
∴ Empirical formula: MCl3
4
Use of the Data Booklet is relevant to this question.
The electronic configurations of four elements are given.
Which element will most easily form an isolated gaseous ion with the charge of
+3?
Answer: C
A 1s22s22p3
Removal of electrons from the 2nd principal quantum shell requires more
energy than removing from the 3rd principal quantum shell.
B 1s22s22p63s23p3
Removal of electrons from the 3rd principal quantum shell requires more
energy than removing electrons from the 4th principal quantum shell.
C 1s22s22p63s23p63d14s2
Both C and D involve removing two 4s electrons and one 3d electron. It
is easier to remove the 3d electron from this element as the electron
experiences less effective nuclear charge attraction.
D 1s22s22p63s23p63d54s2
Removal of one 3d electron from D is harder than from C since the
electron experiences greater effective nuclear charge attraction. There
© ACJC 2015 JC1 Term Exam 2015
3
are more protons in the nucleus and the number of inner shell electrons
is the same (almost constant shielding effect)
5
In an acidic medium, 3 moles of the compound, CaXF4, was found to completely
oxidise 2 moles of FeC2O4, giving the products Fe3+ and CO2.
What is the final oxidation number of element X?
A −1
B 0
C +1
D +2
Answer: B
FeC2O4 → Fe3+ + 2CO2 + 3e−
Amount of electrons lost = 3 x 2 = 6 mol
6
Amount of electrons gained by 1 mol of CaXF4 = =2
3
Initial oxidation number of X = +2
Final oxidation number of X = 2 – 2 = 0
6
When iron is reacted with aqueous iron(III) ions, iron(II) ions are formed.
Assuming the reaction goes to completion, how many moles of Fe and of
Fe3+(aq) would result in a mixture containing equal numbers of moles of
Fe3+(aq) and Fe2+(aq) once the reaction had taken place?
No. of moles No. of moles of
of Fe Fe3+(aq)
A 1 2
B 1 3
C 1 5
D 2 3
© ACJC 2015 JC1 Term Exam 2015
4
Answer: C
[R]: Fe3+ + e− → Fe2+
[O]: Fe → Fe2+
Overall: Fe + 2Fe3+ → 3Fe2+
Fe + 2Fe3+ → 3Fe2+
Initial 1 5 0
Change −1 −2 +3
Final 0 3 3
7 Which element is expected to show the greatest tendency to form covalent
compounds with a non-metallic element?
A Aluminium
B Calcium
C Magnesium
D Sodium
Answer: A
Approach: An ionic compound will form as a result of both elements reacting
together. The degree of covalent character in the ionic compound depends on:
(i) Charge density of the cation
(ii) Polarisability of anion
Al3+ has the highest charge density among the four.
8 The value of pV is plotted against p for 2 gases, A and B, where p is the
pressure and V is the volume of the gas.
pV Gas A
Gas B
2x
0 p
Which of the following could be the identities of the gases?
© ACJC 2015 JC1 Term Exam 2015
5
Gas A Gas B
A 0.5 mol of H2 at 25 ºC 0.5 mol of H2 at 50 ºC
B 0.5 mol of H2 at 25 ºC 1 mol of SO2 at 25 ºC
C 0.25 mol of SO2 at 25 ºC 0.5 mol of H2 at 25 ºC
D 1 mol of SO2 at 25 ºC 0.5 mol of H2 at 25 ºC
Answer: C
From the curve of the graph, students should also observe that that Gas A
deviates more from ideality than Gas B. i.e. Gas A has
Stronger strength of intermolecular forces of attraction, or
Lower temperature
Since pV = nRT, the vertical axis (pV) can also depend on nRT. In other words,
doubling the number of moles causes pV to double, or
doubling absolute temperature also causes pV to double
In option A, lower temperature of Gas B causes it to deviate less from ideality
that Gas A. However, increasing the temperature from 25 ⁰C to 50 ⁰C does not
cause pV to double from x to 2x. In order for the value of pV to double, absolute
temperature (in K) should be doubled.
In option B, Gas A is H2 while Gas B is SO2. However, SO2 has greater strength
of Van der Waals forces because it has pd-pd interactions between molecules
while H2 has id-id interactions between molecules.
In option C, Gas A has stronger Van der Waals forces, hence the graph
deviates more from ideality. There is twice the number of moles of Gas B than
Gas A, hence pV is doubled.
In option D, the identity of the gases corresponds to their deviation from ideality.
However there is twice the number of moles of Gas A than Gas B, and that
contradicts the value of pV in the graph.
9
Rank the following four molecules in ascending boiling points.
V: X:
Y: Z:
© ACJC 2015 JC1 Term Exam 2015
6
A X<V<Y<Z
B Y<V<Z<X
C X<Z<V<Y
D V<Y<X<Z
Answer: D
X and Z have hydrogen bonds between molecules while V and Y have
permanent dipole-permanent dipole interactions between molecules.
Hence the boiling points of X and Z are generally greater than V and Y.
Z and Y are linear while X and V are spherical, causing Z and Y have stronger
instantaneous dipole-induced dipole interactions than X and V respectively.
Ascending order for strength of intermolecular forces of attraction: V < Y < X < Z
Ascending order of boiling points: V < Y < X < Z
10 The diagram represents the melting points of four consecutive elements in the
third period of the Periodic Table.
Melting point / K
0 Proton number
The sketches below represent another two properties of the same elements.
Property M Property N
0 Proton number 0 Proton number
What are properties M and N?
Property M Property N
© ACJC 2015 JC1 Term Exam 2015
7
A Third ionisation energy Electronegativity
B Number of valence electrons Boiling point
C Ionic radius Effective nuclear charge
D Electrical conductivity Atomic radius
Answer: A
Based on the trend of the melting points, student can deduce that the four
consecutive elements are Si, P, S and Cl.
(Examiners’ comments: There are more than one way to solve this MCQ; below
is a suggested method that uses elimination of options)
Since Property N shows an increasing trend,
Option B can be eliminated since boiling point varies from Si to Cl.
Option D can be eliminated since atomic radius decreases from Si to Cl.
Using the trend of Property M, option C can be eliminated because ionic radius
decreases from P3- to Cl-.
Test yourself: Try to sketch the trends of all the properties given in options A to
D.
11 In which set of species is the bond angle of the second species larger than that
of the first one?
A NCl3 , H2O
B BF4− , SCl2
C PCl6− , ClO2−
D Br2O , SF6
Answer: C
PCl6− has an octahedral shape, while ClO2− has a bent shape.
12
Use of the Data Booklet is relevant to this question.
With reference to the following four reactions,
P Br2(g) → 2Br(g)
Q 2Cl(g) → Cl2(g)
R CH3(g) + Cl(g) → CH3Cl(g)
S CH4(g) → CH3(g) + H(g)
the order of enthalpy change from the most exothermic to the most endothermic
would be:
A P→Q→R→S
B Q→R→S→P
© ACJC 2015 JC1 Term Exam 2015
8
C R→Q→P→S
D S→P→Q→R
Answer: C
Equation Bond breaking / forming Magnitude
(kJ mol-1)
P Br2(g) → 2Br(g) Breaking of Br-Br bond +193
Q 2Cl(g) → Cl 2(g) Forming of Cl-Cl bond -244
R CH3(g) + Cl(g) → CH3Cl(g) Forming of C-Cl bond -340
S CH4(g) → CH3(g) + H(g) Breaking of C-H bond +410
Most exothermic to the most endothermic: R → Q → P → S
13 In which of the following process are hydrogen bonds broken?
Answer: B
Bonds / interactions overcomed
A H2(l) → H2(g) Instantaneous dipole-induced dipole
interaction between H2 molecules
B NH3(l) → NH3(g) Hydrogen bonds between NH3
molecules
C 2HI(g) → H2(g) + I2(g) H-I covalent bonds between H and I
atoms
D CH4(g) → C(g) + 4H(g) C-H covalent bonds between C and H
atoms
14 Which of the following equations has a negative enthalpy value?
Answer: D Type of enthalpy Endothermic /
change Exothermic
A Ag+(aq) + Cl−(aq) → Ag+(g) + Cl−(g) −ΔHhyd(Ag+) & Endothermic (note
- that ΔHhyd is
−ΔHhyd(Cl )
negative so −ΔHhyd
is positive)
B Cl2(g) → 2Cl(g) Bond breaking, Endothermic
BDE(Cl-Cl)
C Ag(g) → Ag+(g) + e− 1st ionisation of Endothermic
Ag
D Cl(g) + e− → Cl−(g) 1st Electron Exothermic
affinity of Cl
15 When steam condenses, 44 kJ mol−1 of heat enthalpy is evolved.
What is the entropy change when 54 g of steam condenses at 100 oC?
© ACJC 2015 JC1 Term Exam 2015
9
A −354 J K−1
B −118 J K−1
C −1320 J K−1
D +354 J K−1
Answer: A
When steam condenses, 44kJ of heat are given off for every mole of H2O.
54
54 g of steam is = 3.0 mol of H2O Hence ΔH = − ( 3 x 44) kJ
18
Using ΔG = ΔH − TΔS where ΔG = 0
ΔS = ΔH / T = −( 3 x 44 x 103)/373 = − 354 JK−1
16
The enthalpy change of solution of magnesium fluoride is negative (−17.7 kJ mol−1),
but the enthalpy change of solution of barium fluoride is positive (+3.8 kJ mol−1).
What conclusion can be drawn from these data?
1 The lattice energy of barium fluoride is more exothermic than the sum of the
hydration energies of the barium and fluoride ions.
2 The lattice energy of barium fluoride is more exothermic than that of
magnesium fluoride.
3 The hydration energy of the barium ion is more exothermic than that of the
magnesium ion.
Answer: D
ΔH soln = ΔH hydration – LE
As LE and ΔH hydration are always exothermic, ΔH soln will be endothermic if LE >
ΔH hydration and exothermic if ΔH hydration > LE.
In the case of BaF2, statement 1 implies that the ΔH hydration < LE. Hence ΔH soln
of BaF2 would be +ve.
Statement 2 is incorrect as the LE of MgF2 is larger than that of BaF2 ,
since LE α (q+q-)/(r+ + r-); q+, q- and r- are the same, but r+ is larger for Ba2+.
Statement 3 is incorrect as ΔH hydration ∝ q+/r+. Since r+ for Ba2+ is larger, ΔH
hydration of Ba2+ is lower.
17 Which of the following is/are reason(s) why the first ionisation energy of neon is
higher than that of fluorine?
1 The nuclear charge in neon is greater than that in fluorine.
2 Neon has a complete octet, but fluorine does not.
3 The atomic radius of fluorine is less than that of neon.
© ACJC 2015 JC1 Term Exam 2015
10
Answer: D
Statement 1 is correct. The Effective Nuclear charge for Ne is higher, hence
attraction for valence electrons is stronger, first IE of Ne is higher.
Statement 2 – a complete octet does not imply that the first IE will be higher – the
nuclear charge affects the IE more directly.
Statement 3 – As nuclear charge increases across a period, Ne is expected to have
a smaller atomic radius.
18 Which of the following solid(s) contain more than one type of bond?
1 Brass (an alloy of copper and zinc)
2 Graphite
3 Ice
Answer: C
Statement 1 : Brass contains Zn and Cu – both are metals – so they have only
metallic bonds.
Statement 2 : Graphite has covalent bonds between the C atoms in a layer and
van der Waals forces of attraction between the hexagonal layers.
Statement 3 : Ice has covalent bonds between O and H atoms in H2O and
hydrogen bonds between the H2O molecules.
19
The following represents the electronic configuration of both a Group II cation and
a Group VII anion.
1s22s22p63s23p63d104s24p6
The radius of the anion is approximately twice that of the cation.
Which reason(s) explain the difference in radius?
1 The cation has a greater nuclear charge than the anion.
2 On forming the anion from its atom, the extra electron repulsion makes the ion
much larger.
3 There is more shielding in the anion than in the cation.
Answer: B
There is a total of 36 electrons. For a Gp II cation to have 36 electrons, it has lost 2
electrons. So the Gp II element has 38 electrons and 38 protons.
The Gp VII anion has 36 electrons, the element has gained 1 electron. So it has 35
electrons and 35 protons.
© ACJC 2015 JC1 Term Exam 2015
11
Statement 1 : Hence, the cation has a higher nuclear charge than the anion.
Statement 2 – For the Gp VII anion, inter electronic repulsion between the added
electron and the unpaired electron would cause the ionic radius to be larger than
the atomic radius.
Statement 3 – the anion and cation have the same electron configuration, hence
they have the same number of shielding electrons. Hence shielding effect is the
same.
20 Which of the following would be required to determine the lattice energy of AlF3 in
a Born-Haber cycle?
1 First electron affinity of fluorine
2 Enthalpy change of atomisation of AlF3
3 Bond energy of Al – F
Answer: D
The Born Haber Cycle relates the LE (formation of AlF3(s) from its gaseous ions)
and the ΔH formation of AlF3 (from its elements under standard conditions).
Statement 1 : Hence, first electron affinity is needed to change the F(g) to F−(g).
Statement 2 : There is no need to atomise the AlF3, only atomization of the
elements Al(s) and F2(g) are required.
Statement 3 : Bond Energies are not needed in the Born Haber Cycle.
© ACJC 2015 JC1 Term Exam 2015
12
Section B (25 marks)
Answer all questions in the spaces provided on the question paper.
1 Planning
A solution FA1 containing 75.0 g dm−3 of FeSO4.xH2O has been prepared. To
determine the water of crystallisation, x, FA1 was to be titrated against a
standard solution of acidified KMnO4.
A standard solution FA2 having the concentration of 1.12 mol dm−3 KMnO4 was
prepared.
A student carried out a preliminary test of the titration and it was found that 20
drops of FA1 required 4 drops of FA2 for complete reaction. Based on this
preliminary test, the student decided that FA2 must be diluted before it could be
used for titration. The diluted solution would be named FA3.
(a) Calculate the concentration of FA3 given that the titre volume must be
approximately equal to the volume of the sample pipetted.
To have a titre reading that is approximately 25 cm3,
5 4
Concentration of diluted KMnO4 = 25 X 1.12 (or 20 X 1.12)
= 0.224 mol dm-3
Comments:
-Recognise that FA2 is too concentrated and needs to be diluted.
-Recognise for the volume of FA1 used to increase from 4 to 20 drops, its
concentration must decrease by a factor of 5.
(b) Give a step-by-step description of how the experiment would be carried
out. Your plan should be prescriptive and include details of
Appropriate apparatus with dimensions
Calculations for the volume of FA2 required
How you would prepare a solution of FA3
A titrimetric method to carry out sufficient titrations to allow an
accurate end-point to be obtained
0.224 x 250 = 1.12 x volume of FA2 required
volume of FA2 required = 50.00 cm3
1. Measure 50.00 cm3 of FA2 from a burette into a 250 cm3
volumetric flask.
2. Top up the volumetric flask to the marking using deionized water.
3. Stopper and shake well to obtain a homogeneous solution.
4. Pipette 25.0 cm3 of FA1 into a 250 cm3 conical flask.
5. Fill a 50 cm3 burette with FA3.
6. Titrate FA1 using FA3 until one drop of FA3 turn the solution from
pale yellow to a permanent pink colour.
7. Repeat the titration to obtain consistent readings of 0.10cm3.
© ACJC 2015 JC1 Term Exam 2015
13
Comments:
- pH indicators are usually not used in redox titrations, since there is no
sharp change in pH.
- When one solution (KMnO4) is intensely coloured, this solution may be
used to indicate the end-point of titration.
- Some stated the colour change to be purple to colourless, where the
original content of the conical flask is actually Fe2+ instead of MnO4−.
2 (a) The successive ionisation energies of a period 2 element, X, are given in
the table. Explain which group the element X belongs to.
Successive
ionisation
1086 2352 4620 6222 37831 47277
energies /
kJ mol-1
There is a large increase in IE from the removal of the fourth to fifth
electron. This shows that the fifth electron is from the inner
quantum shell and there are 4 valence electrons, hence X is in
group IV.
Comments:
- Students should avoid ambiguous terms such as “going to the next
shell”.
- Some stated Group 4 instead of Group IV.
(b) When atoms which have been completely stripped of their electrons are
passed through two plates carrying electric charge, the nuclei of element
X and another nuclei of element Y, from the same group, are deflected
as follows.
2⁰ Y
1.763⁰
Deduce the identity of element Y.
Angle of deflection = k (charge / mass)
2⁰ = k (6 / 12)
k=4
© ACJC 2015 JC1 Term Exam 2015
14
For element Y,
Angle of deflection = 4 x (charge / mass)
Charge / mass = 1.763⁰ / 4 = 0.44075
Hence element Y is Ge (32 / 72.6 = 0.44077)
Alternative:
(C1/M1) / Angle1 = (C2/M2) / Angle2
(6/12) / 2 = (C2/M2) / 1.763
C2/M2 = 0.4407
Comments:
- Some did not read carefully that Y is in the same group as X.
(c) Write the electronic configuration of the element Y in (b).
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2
Comments:
3 (a) Chlorine perchlorate is an unstable oxide of chlorine. It decomposes
readily to form oxygen, chlorine and solid dichlorine hexoxide, Cl2O6.
a b
Chlorine perchlorate
(i) State the oxidation state of each chlorine atom in chlorine
perchlorate.
Cla : +7 Clb : +1
Comments:
- Many students did not appreciate the relevance of the molecule’s
structure to the oxidation state of Cl.
- Some students gave negative values when Cl is the less
electronegative element in the compound.
(ii) Write a balanced equation for the decomposition of chlorine
perchlorate, and state the type of reaction.
2 Cl2O4 → O2 + Cl2 + Cl2O6
Disproportionation reaction
Comments:
- Student were careless and thought that Cl2O6 was the compound
that was undergoing decomposition.
- Students should not mention redox as disproportionation is more
specific than redox.
© ACJC 2015 JC1 Term Exam 2015
15
(b) When a sample of chlorine perchlorate was allowed to decompose, the
products occupied 210.0 cm3 at the pressure of 9.50 x 104 Pa and the
temperature of 333 ⁰C.
(i) State the two assumptions that need to be made in order for the
gas mixture to exhibit ideal behavior.
The volume of the gas molecules are negligible compared to
the volume of the gas.
The intermolecular forces of attraction are negligible.
Comments:
- Give the 2 most important assumptions that ensure ideal gas
behavior.
- Do not mention “no intermolecular forces of attraction”.
- A few stated “the volume of the gas molecules are negligible”
without making a comparison to the volume of the gas as a whole.
(ii) By assuming ideal gas conditions, calculate the mass of chlorine
perchlorate that decomposed.
𝑝𝑉
= 𝑛
𝑅𝑇
9.50 × 104 × 210 × 10−6
= 𝑛
8.31 × (333 + 273)
n = 0.003962 mol
Mass of chlorine perchlorate
= 0.003962 x (2 x 35.5 + 4 x 16.0)
= 0.535 g (3 s.f.)
Comments:
- Many students do not convert the volume of cm3 to m3 or they
have the wrong conversion.
- A few did not convert the temperature given to Kelvin.
- The amount calculated is the amount of gas present in the
mixture.
4 (a) (i) 10.0 g of Cr2(SO4)3 crystals were mixed into 50 cm3 of water and
stirred until the solid completely dissolved. It was found that the
temperature fell by 5.9 °C.
Given that the experimental setup has 90% efficiency, calculate the
enthalpy change of solution of Cr2(SO4)3. Assume the specific heat
capacity of water to be 4.18 J K−1 g−1.
Heat lost by water = 50 x 4.18 x 5.9
© ACJC 2015 JC1 Term Exam 2015
16
= 1233.1 J
Heat gained by solvation of Cr2(SO4)3
= 1233.1 / 90 x 100
= 1370.1 J
No. of moles of Cr2(SO4)3 dissolved = 10.0 / 392.3
= 0.02549 mol
Enthalpy change of solution of Cr2(SO4)3
= + 1370.1 / 0.02549
= + 53.8 kJ mol−1 (3 s.f.)
Comments:
- Some students did not understand the term “90% efficiency” and
multiplied the heat lost by water by 0.9.
- Students must remember to include the sign and the units for the
enthalpy change of solution.
- Some students also used the mass of the Cr2(SO4)3 crystals (10g)
instead of the mass of water in the calculation of the heat lost by
water.
- Students must write clear statements to illustrate their working.
(ii) The following energy level diagram shows the heat content of
Cr2(SO4)3 under various conditions.
Using your value from part (a)(i), calculate the lattice energy of the
compound.
Enthalpy / kJ mol−1
2Cr3+ (g) + 3SO42− (g)
Hydration energy = −2380 kJ mol−1
Lattice
energy
2Cr3+ (aq) + 3SO42− (aq)
∆Hsol
Cr2(SO4)3 (s)
ΔHsol = HE – LE
LE = −2380 – (+53.8)
= −2430 kJ mol−1 (3 s.f.)
Comments:
- Students should use the diagram provided to determine the lattice
© ACJC 2015 JC1 Term Exam 2015
17
energy, as well as its relation to hydration energy and ∆Hsol. (some
gave the wrong equation when relating the 3 terms together)
- The diagram provides a hint to ∆ Hsol being an endothermic
process.
(iii) Justify the sign of hydration energy of Cr2(SO4)3 by sketching a
diagram of a Cr3+ ion and water molecules in the aqueous solution.
In your diagram, name the interaction involved.
+ H
O H
- +
Cr3+
ion-dipole interaction
Comments:
- Students need to draw water molecules correctly and include the
dipoles for O and H atom.
- Some students mentioned about permanent dipole and
permanent dipole interactions.
(b) The standard entropy change of solution of Cr2(SO4)3 is −287 J mol−1 K−1.
Suggest a reason for the negative sign of its entropy value.
Entropy decreases because several water molecules are arranged in
an orderly manner around each aqueous ion. [1]
Comments:
- A lot of answers explained the increase in disorder due to the
dissolution process but this involves a positive entropy change.
- Students confused entropy with enthalpy.
- Students misunderstood that a negative ΔS meant that the system was
more disordered.
- Students did not understand the process of solution and talked about
gaseous ions.
5 (a) Draw the dot and cross diagram for CS2 and NO.
S C S N O
Comments:
- Some answers had more than 8 electrons around N, C or O.
© ACJC 2015 JC1 Term Exam 2015
18
- Some students drew circles (illustrating orbitals) for the dot and cross
diagram.
(b) Using your answer in (a), draw the dot and cross diagram for N2O2 which
occurs in solid NO.
O N
N O
Comments:
- Students are to recognise N2O2 as a dimer of NO.
- Lone electron of N in NO will be involved in bonding with each other.
(c) State the shape about the N atoms in (b).
Shape: Bent
Comments:
(d) Explain why the melting point of CS2 is higher than that of NO.
NO and CS2 have simple molecular structure.
Electron cloud of CS2 is larger than NO and more polarisable .This
results in stronger induced dipole – induced dipole forces of
attraction between CS2 molecules which require more energy to be
broken, resulting in a higher melting point for CS2.
Comments:
- Some students thought CS2 is a polar molecular but did not recognise
that the polarity of the bond cancelled each other.
- Some answers discussed the shape of the electron cloud, with CS2
having a more elongated one. However, the sizes of electron cloud for
both molecules are different. There is no basis for comparing shape of
electron cloud.
- Some students talked about intramolecular bonding instead of
intermolecular bonding. Students must recognise that both CS2 and NO
are simple molecules. Melting involves the breaking of intermolecular
bonds between the molecules.
© ACJC 2015 JC1 Term Exam 2015
19
Section C (15 marks)
1 PF3 is a highly toxic compound that binds to haemoglobin in blood in a
similar fashion to CO. Below is some information about PF3 and one of its
constituent element, phosphorus.
Enthalpy change of formation of PF3(g) – 945 kJ mol−1
Enthalpy change of atomisation of P4(s) + 314.6 kJ mol−1
(a) (i) Define the term standard enthalpy change of atomisation.
It is the energy required to produce one mole of gaseous
atoms from its element under standard conditions.
Comments:
- Some students did not include standard conditions and wrote
standard state instead.
- Answers that wrote “energy change / energy involved” were not
accepted as the atomisation process always absorbs energy.
(ii) Write an equation, using P4(s) as an example, to illustrate your
answer in (a)(i).
1
P4(s) P(g)
4
Comments:
(b) Using information from the Data Booklet and that provided above,
construct an energy cycle and calculate the bond energy of the P – F
bond in PF3(g).
∆Hf
P4(s) + F2(g) PF3(g)
∆Hatom BE (F – F) 3 BE (P – F)
P(g) + 3F(g)
∆Hatom + 3/2 BE (F – F) − ∆Hf = 3 BE (P – F)
BE (P – F) = +499 kJ mol−1
Comments:
- Some students did not know how to draw the energy cycle or did not
label the energy cycle correctly.
- State symbols must be included in the energy cycle for marks to be
awarded
© ACJC 2015 JC1 Term Exam 2015
20
(c) (i) Calculate ∆G for the reaction at 25 °C.
1 3
P4 (s) + F2 (g) PF3 (g) ∆S = − 72.2 J mol−1 K−1
4 2
∆G = – 945 – (298)(– 0.0722)
= – 923 kJ mol−1
Comments:
- Some students forgot to divide ∆S by 1000.
(ii) State the spontaneity of the reaction in (c)(i).
Reaction is spontaneous.
Comments:
(iii) State the maximum amount of free energy that can be obtained
when 0.5 mol of PF3 is formed from the reaction in (c)(i).
462 kJ
Comments:
- Many gave the answer −462 kJ.
(d) Both the elements, nitrogen and phosphorus lie in Group V. Nitrogen
exists as N2 molecules while phosphorus exists as P4 molecules, with
its structure shown below.
(i) Suggest why phosphorus does not form molecules of P2.
The atomic orbitals of P are larger than N. This results in
ineffective / poor side – on overlap of the orbitals when P
forms a triple bond with another atom.
Comments:
- Some students commented that it was an unstable molecule
which is not accepted as this merely rephrases the question.
- Students need to recognize that in P2, both atoms bond via a
triple bond (similar to N≡N in N2). This bond is rather weak
(making P2 reactive / unstable) and students need to explain why
the triple bond is weak.
© ACJC 2015 JC1 Term Exam 2015
21
(ii) With reference to the structures of PF3 and AlF3, explain the large
difference in their melting points.
PF3 exists as simple molecules / has a simple molecular
structure. AlF3 has a giant ionic lattice structure. Weak
permanent dipole – permanent dipole forces of attraction
exist between the molecules of PF3 while strong electrostatic
forces of attraction are present between the ions in AlF3,
requiring larger amounts of energy to overcome. This results
in AlF3 having a higher melting point.
Comments:
- A few erroneously thought that AlF3 has a simple molecular
structure (like AlCl3).
(iii) Both NF3 and PF3 are soluble in polar solvents but PF5 is
insoluble. Explain why PF5 is insoluble in polar solvents.
PF5 is non polar and induced dipole – induced dipole forces
of attraction are the predominant forces of attraction
between its molecules. It is not able to form favourable
intermolecular forces of attraction with solvent molecules /
overcome the stronger permanent dipole – permanent dipole
forces of attraction between the solvent molecules.
Comments:
- Some answers only described PF5 as being non-polar and
therefore could not dissolve in polar solvents. This was
insufficient, as a further elaboration on the nature of the forces of
attraction (id-id) between PF5 molecules was needed. This is then
associated to the inability of PF5 to break the stronger pd-pd
interactions between solvent molecules.
- Some only made a specific reference to water instead of polar
solvents in general. As a result, their answers were along the
lines of PF5 not being able to form hydrogen bonding with water,
which is insufficient for this question.
- Answers that focused on addressing the reason for NF3 and
PF3’s ability to dissolve well in polar solvents did not answer the
question.
- Some students discussed the reasons why PF5 is non polar
which was not the focus of the question.
© ACJC 2015 JC1 Term Exam 2015
22
(iv) When PF3 reacts with NH3, a single product is formed. Draw the
structure of this product, showing clearly the geometry about the
P and N atoms.
Comments:
- Dative bonding, with the P atom of PF3 being the electron donor,
is not possible as N cannot expand its octet.
- Some students forgot the lone pair on P when determining its
geometry.
- Some did not read the question carefully, giving a 2D structure
without mentioning the shape around the P and N atoms.
© ACJC 2015 JC1 Term Exam 2015
You might also like
- 638b7346d28d9Document20 pages638b7346d28d9HC GamerNo ratings yet
- Y11 Chemistry Diagnostic TestDocument17 pagesY11 Chemistry Diagnostic TestTaiba MusaNo ratings yet
- © Ucles 2007 9701/01/o/n/07Document13 pages© Ucles 2007 9701/01/o/n/07Adil ArifNo ratings yet
- 2021 H2 Chemistry Prelim Paper 1Document15 pages2021 H2 Chemistry Prelim Paper 1clarissa yeoNo ratings yet
- NL MCQ Timed Practice 14 (R06)Document4 pagesNL MCQ Timed Practice 14 (R06)Alvin LeeNo ratings yet
- Cambridge IGCSE: Chemistry 0620/11Document16 pagesCambridge IGCSE: Chemistry 0620/11صالح ابراهيمNo ratings yet
- June 2005 QP - Paper 1 CIE Chemistry IGCSEDocument16 pagesJune 2005 QP - Paper 1 CIE Chemistry IGCSEMedo O. EzzatNo ratings yet
- June 2022 (v2) QP - Paper 2 CAIE Chemistry IGCSEDocument16 pagesJune 2022 (v2) QP - Paper 2 CAIE Chemistry IGCSEKaise AlloudamiNo ratings yet
- ASRJC H2 Chem 2021 P1 QPDocument20 pagesASRJC H2 Chem 2021 P1 QPantesipation ฅ'ω'ฅNo ratings yet
- NL MCQ Challenge 03Document5 pagesNL MCQ Challenge 03Alvin Lee100% (1)
- June 2003 QP - Paper 1 CIE Chemistry IGCSEDocument20 pagesJune 2003 QP - Paper 1 CIE Chemistry IGCSEMedo O. EzzatNo ratings yet
- HW Bonding Past Paper QuestionsDocument5 pagesHW Bonding Past Paper QuestionsyijinrobloxNo ratings yet
- Test 2 Metal With AnswerDocument5 pagesTest 2 Metal With AnswerIsaacNo ratings yet
- Cambridge IGCSE: Chemistry 0620/22Document16 pagesCambridge IGCSE: Chemistry 0620/22mercy.cyfn.109No ratings yet
- D Block Live Class-2 Teacher NotesDocument32 pagesD Block Live Class-2 Teacher NotesANo ratings yet
- Year End Paper 1 ChemDocument9 pagesYear End Paper 1 ChemNOR ATIKAH BINTI TAKRUDDIN MoeNo ratings yet
- 0620 s16 QP 12 PDFDocument12 pages0620 s16 QP 12 PDFSiying LaiNo ratings yet
- 2020 Sec 4 Pure Chemistry SA2 Ngee Ann SecondaryDocument35 pages2020 Sec 4 Pure Chemistry SA2 Ngee Ann SecondaryDORA SIN YU KWOKNo ratings yet
- TPJC H2chem Prelim p1 2009Document14 pagesTPJC H2chem Prelim p1 2009Amos YapNo ratings yet
- ASRJC 2022 J2 H2Prelim P1 QPDocument16 pagesASRJC 2022 J2 H2Prelim P1 QPqiyunNo ratings yet
- 2014 H2 Chem Promo (DHS) - PKDocument37 pages2014 H2 Chem Promo (DHS) - PKdragon slayerNo ratings yet
- Answer All Questions.: Section A (15 Marks)Document7 pagesAnswer All Questions.: Section A (15 Marks)chee pin wongNo ratings yet
- Cambridge IGCSE: Chemistry 0620/22Document16 pagesCambridge IGCSE: Chemistry 0620/22Titan XosmosNo ratings yet
- Complete Inorganic MarathonDocument407 pagesComplete Inorganic MarathonAdithya kumar JhaNo ratings yet
- Review SheetDocument15 pagesReview Sheetmtayyab zahidNo ratings yet
- IGCSE Pass PaperDocument20 pagesIGCSE Pass PaperNgoc Quang NguyenNo ratings yet
- Cambridge IGCSE: Chemistry 0620/21Document16 pagesCambridge IGCSE: Chemistry 0620/21Mina AbdouNo ratings yet
- IGCSE May-Jun 2021 Chemistry Paper 21Document16 pagesIGCSE May-Jun 2021 Chemistry Paper 21Aditya SenthilNo ratings yet
- 0620 - s05 - QP - 1.chemistry O LevelDocument16 pages0620 - s05 - QP - 1.chemistry O LeveltiffaNo ratings yet
- NL MCQ Challenge 02Document5 pagesNL MCQ Challenge 02Alvin LeeNo ratings yet
- Inorganic Chemistry AssignmentDocument11 pagesInorganic Chemistry AssignmentK VIKASNo ratings yet
- Cambridge IGCSE: Chemistry 0620/23Document16 pagesCambridge IGCSE: Chemistry 0620/23jpfornicolaNo ratings yet
- B10 P2 ChemistryDocument14 pagesB10 P2 Chemistryegi.academicinfoNo ratings yet
- Grade 9 MCQDocument12 pagesGrade 9 MCQBoringNo ratings yet
- Cambridge IGCSE: Chemistry 0620/22Document16 pagesCambridge IGCSE: Chemistry 0620/22afyNo ratings yet
- Cambridge IGCSE: Chemistry 0620/23Document16 pagesCambridge IGCSE: Chemistry 0620/23SasukeNo ratings yet
- Section A (Question) FINALDocument8 pagesSection A (Question) FINALcalderteoNo ratings yet
- University of Cambridge International Examinations International General Certificate of Secondary Education Chemistry Paper 1 Multiple Choice October/November 2006 45 MinutesDocument16 pagesUniversity of Cambridge International Examinations International General Certificate of Secondary Education Chemistry Paper 1 Multiple Choice October/November 2006 45 MinutesVarun PanickerNo ratings yet
- 2016 P 1Document5 pages2016 P 1Fomukwin Ayenui NoelNo ratings yet
- Chemical Bonding MCQ 16Document4 pagesChemical Bonding MCQ 16sunNo ratings yet
- 2021 EJC JC2 Prelim H2 Chemistry Paper 1 QPDocument10 pages2021 EJC JC2 Prelim H2 Chemistry Paper 1 QPclarissa yeoNo ratings yet
- 0620 s05 QP 1 - (2) 1Document16 pages0620 s05 QP 1 - (2) 1arfahnadeem42No ratings yet
- Chemical Bonding - Practice Sheet - JEE ChallengersDocument5 pagesChemical Bonding - Practice Sheet - JEE ChallengerssadatarbabedNo ratings yet
- Cambridge IGCSE: Chemistry 0620/23Document16 pagesCambridge IGCSE: Chemistry 0620/23...No ratings yet
- 4024q1 Specimen PaperdocxDocument12 pages4024q1 Specimen PaperdocxLeses MayNo ratings yet
- Final HSSC-I Chemistry Model Paper MergedDocument10 pagesFinal HSSC-I Chemistry Model Paper MergeddasddaNo ratings yet
- Kimia T4 2023 - DLPDocument10 pagesKimia T4 2023 - DLPbrendan chee junNo ratings yet
- Cambridge IGCSE: Chemistry 0620/23Document16 pagesCambridge IGCSE: Chemistry 0620/23mercy.cyfn.109No ratings yet
- Cambridge IGCSE: Chemistry 0620/23Document16 pagesCambridge IGCSE: Chemistry 0620/23mostafa barakatNo ratings yet
- MCQ 1Document15 pagesMCQ 1Nissa AECNo ratings yet
- Exam 5 May 2021 21 2023-11-04 21 - 42 - 39Document16 pagesExam 5 May 2021 21 2023-11-04 21 - 42 - 39fakeeamail999773No ratings yet
- FSM Neet 2 PDFDocument50 pagesFSM Neet 2 PDFSuyash Dahake100% (2)
- Cambridge IGCSE: Chemistry 0620/23Document16 pagesCambridge IGCSE: Chemistry 0620/23shabanaNo ratings yet
- Cambridge IGCSE: Chemistry 0620/22Document16 pagesCambridge IGCSE: Chemistry 0620/22ZubairHassanNo ratings yet
- North Vista 2015 Prelim Paper 1Document20 pagesNorth Vista 2015 Prelim Paper 1GM MonsterEtaNo ratings yet
- SRJC Promo 2009 Paper 1Document16 pagesSRJC Promo 2009 Paper 1gretchen92No ratings yet
- Cambridge IGCSE: Chemistry 0620/22Document16 pagesCambridge IGCSE: Chemistry 0620/22Rita SmairatNo ratings yet
- Exam 7 - Paper 2 (Model 2)Document16 pagesExam 7 - Paper 2 (Model 2)m.altokhy07No ratings yet
- 0620 m21 QP 22 MergedDocument132 pages0620 m21 QP 22 Mergedchangemakergrade8No ratings yet
- 04 03 Mass Spectrometry 12 PDFDocument8 pages04 03 Mass Spectrometry 12 PDFisimone7No ratings yet
- H2 - MBT - Revision Package - Integration - SolutionsDocument4 pagesH2 - MBT - Revision Package - Integration - SolutionsTimothy HandokoNo ratings yet
- H2 MBT Revision Package Functions SolutionsDocument2 pagesH2 MBT Revision Package Functions SolutionsTimothy HandokoNo ratings yet
- March Block Test Timed Trial 3 - SolutionsDocument4 pagesMarch Block Test Timed Trial 3 - SolutionsTimothy HandokoNo ratings yet
- March Block Test Timed Trial 1 - SolutionsDocument14 pagesMarch Block Test Timed Trial 1 - SolutionsTimothy HandokoNo ratings yet
- March Block Test Timed Trial 2 - SolutionsDocument15 pagesMarch Block Test Timed Trial 2 - SolutionsTimothy HandokoNo ratings yet
- Asset-V1 UTokyoX+UTokyo007x+1T2018+Type@Asset+Block@Unit 17 Applications of SpectrophotometryDocument8 pagesAsset-V1 UTokyoX+UTokyo007x+1T2018+Type@Asset+Block@Unit 17 Applications of SpectrophotometryTimothy HandokoNo ratings yet
- March Block Test Timed Trial 3 - SolutionsDocument14 pagesMarch Block Test Timed Trial 3 - SolutionsTimothy HandokoNo ratings yet
- H2 MBT Revision Package Inequalities SolutionsDocument3 pagesH2 MBT Revision Package Inequalities SolutionsTimothy HandokoNo ratings yet
- MYE Timed Trial 2 - SolutionsDocument13 pagesMYE Timed Trial 2 - SolutionsTimothy HandokoNo ratings yet
- JC2 H2 Mathematics (9758) Revision Package Arithmetic and Geometric Progressions Suggested SolutionsDocument2 pagesJC2 H2 Mathematics (9758) Revision Package Arithmetic and Geometric Progressions Suggested SolutionsTimothy HandokoNo ratings yet
- H2 MYE Revision Package Techniques of Differentiation SolutionsDocument5 pagesH2 MYE Revision Package Techniques of Differentiation SolutionsTimothy HandokoNo ratings yet
- H2 MBT Revision Package Differential Equations SolutionsDocument3 pagesH2 MBT Revision Package Differential Equations SolutionsTimothy HandokoNo ratings yet
- H2 MBT Revision Package Complex Numbers SolutionsDocument3 pagesH2 MBT Revision Package Complex Numbers SolutionsTimothy HandokoNo ratings yet
- H2 MYE Revision Package Vectors SolutionsDocument11 pagesH2 MYE Revision Package Vectors SolutionsTimothy HandokoNo ratings yet
- MYE Timed Trial 1 - SolutionsDocument15 pagesMYE Timed Trial 1 - SolutionsTimothy HandokoNo ratings yet
- H2 MYE Revision Package Functions SolutionsDocument10 pagesH2 MYE Revision Package Functions SolutionsTimothy HandokoNo ratings yet
- H2 MYE Revision Package Discrete Random Variables SolutionsDocument7 pagesH2 MYE Revision Package Discrete Random Variables SolutionsTimothy HandokoNo ratings yet
- Eog GH8 AecDocument971 pagesEog GH8 AecTimothy HandokoNo ratings yet
- H2 MYE Revision Package Hypothesis Testing SolutionsDocument9 pagesH2 MYE Revision Package Hypothesis Testing SolutionsTimothy HandokoNo ratings yet
- H2 - MYE - Revision Package - Graphing Techniques - SolutionsDocument9 pagesH2 - MYE - Revision Package - Graphing Techniques - SolutionsTimothy HandokoNo ratings yet
- H2 MYE Revision Package Differentiation SolutionsDocument10 pagesH2 MYE Revision Package Differentiation SolutionsTimothy HandokoNo ratings yet
- H2 MYE Revision Package Integration SolutionsDocument9 pagesH2 MYE Revision Package Integration SolutionsTimothy HandokoNo ratings yet
- Biology: Genetics & Inheritance III: Genetic Basis For Variation IDocument27 pagesBiology: Genetics & Inheritance III: Genetic Basis For Variation ITimothy HandokoNo ratings yet
- Organisation of Genomes - Eukaryotes: Non-Dividing Cells Dividing CellsDocument17 pagesOrganisation of Genomes - Eukaryotes: Non-Dividing Cells Dividing CellsTimothy HandokoNo ratings yet
- Biology: Energy & Equilibrium: RespirationDocument28 pagesBiology: Energy & Equilibrium: RespirationTimothy HandokoNo ratings yet
- H2 - MYE - Revision Package - Binomial Distribution - SolutionsDocument3 pagesH2 - MYE - Revision Package - Binomial Distribution - SolutionsTimothy HandokoNo ratings yet
- H2 MYE Revision Package Differential Equations SolutionsDocument6 pagesH2 MYE Revision Package Differential Equations SolutionsTimothy HandokoNo ratings yet
- 7 GN NF 2 HijDocument629 pages7 GN NF 2 HijTimothy HandokoNo ratings yet
- EYr 6 HQQSBDocument1 pageEYr 6 HQQSBTimothy HandokoNo ratings yet
- Presión de Vapor en LitioDocument4 pagesPresión de Vapor en Litiofernando_johannNo ratings yet
- Module 1.2Document42 pagesModule 1.2jishnushankarNo ratings yet
- Diamond and CBN English 07Document26 pagesDiamond and CBN English 07Vk PrabakranNo ratings yet
- Topic 9 Past Papers Questions With Mark SchemeDocument60 pagesTopic 9 Past Papers Questions With Mark SchemeQasim PerachaNo ratings yet
- SF 0010 0 Metco 320NS PDFDocument8 pagesSF 0010 0 Metco 320NS PDFMehdi KoneshlouNo ratings yet
- Ceratizit ToolsDocument340 pagesCeratizit ToolsguimaslipaNo ratings yet
- Ceramic Ceremet PCBN and PCDDocument48 pagesCeramic Ceremet PCBN and PCDhsvjvv37No ratings yet
- Fabrication of Ceramic Matrix CompositesDocument16 pagesFabrication of Ceramic Matrix CompositesVISION GAMINGNo ratings yet
- Element Six Metalworking-BrochureDocument18 pagesElement Six Metalworking-BrochureGuilherme TrettelNo ratings yet
- Scaffolds QS - Nanotubos de Nitruro de BoroDocument8 pagesScaffolds QS - Nanotubos de Nitruro de BoroCamiloSilvaNo ratings yet
- Metco - Thermal Spray Powders (Nickel Chromium Alloy Boron Nitride)Document3 pagesMetco - Thermal Spray Powders (Nickel Chromium Alloy Boron Nitride)Hamed AhadiNo ratings yet
- B-21-06367 KMT BN9000 BN Lite-1.6 Datasheetpdf-1Document1 pageB-21-06367 KMT BN9000 BN Lite-1.6 Datasheetpdf-1asifNo ratings yet
- Hard Turning AISI 4340 High Strength Low Alloy Steel and AISI D2 Cold Work Tool Steel PDFDocument8 pagesHard Turning AISI 4340 High Strength Low Alloy Steel and AISI D2 Cold Work Tool Steel PDFSinan ChenNo ratings yet
- Microstructure and Mechanical Properties of Al2024-B4C-hBN Reinforced Metal Matrix CompositesDocument5 pagesMicrostructure and Mechanical Properties of Al2024-B4C-hBN Reinforced Metal Matrix CompositesIJRASETPublicationsNo ratings yet
- BN Grades AC6043 and BIN77.InddDocument2 pagesBN Grades AC6043 and BIN77.Inddabir93No ratings yet
- Manyali MSC Thesis PDFDocument109 pagesManyali MSC Thesis PDFGeoff De KlerkNo ratings yet
- Hongling Li 2015Document9 pagesHongling Li 2015Anjali BishtNo ratings yet
- Escholarship UC Item 7hd8r1ftDocument26 pagesEscholarship UC Item 7hd8r1ftAvadhoot RajurkarNo ratings yet
- Best Paper For ResearchDocument7 pagesBest Paper For ResearchHumaira KhalilNo ratings yet
- Solutions-Manual-For-Essentials-Of-Materials-Science-And-Engineering-Si-Edition-3rd-Edition-By-Askeland CHAPTER2Document10 pagesSolutions-Manual-For-Essentials-Of-Materials-Science-And-Engineering-Si-Edition-3rd-Edition-By-Askeland CHAPTER2Lourdes Marie Rosalejos OrtegaNo ratings yet
- High Performance Machining For DielMold Manufacturing - R&D in ProgressDocument22 pagesHigh Performance Machining For DielMold Manufacturing - R&D in ProgressJairo WilsonNo ratings yet
- KJK CatalogueDocument24 pagesKJK CatalogueAslamNo ratings yet
- CeramicsDocument39 pagesCeramicsraja keshavNo ratings yet
- Boron NitrideDocument18 pagesBoron NitrideFulgaNo ratings yet
- Influence of Sequential Surface Treatment Processes On Tribological Performance of Vanadis 6 Cold Work Tool SteelDocument12 pagesInfluence of Sequential Surface Treatment Processes On Tribological Performance of Vanadis 6 Cold Work Tool SteelNaresh PoppathiNo ratings yet
- Essentials of Materials Science and Engineering Si Edition 3rd Edition Askeland Solutions ManualDocument11 pagesEssentials of Materials Science and Engineering Si Edition 3rd Edition Askeland Solutions Manualjeffreyhayesagoisypdfm100% (13)
- Annealing Free, Clean Graphene Transfer Using Alternative Polymer ScaffoldsDocument46 pagesAnnealing Free, Clean Graphene Transfer Using Alternative Polymer ScaffoldsNia SyafiqqNo ratings yet
- Powder Metallurgy Diamond Tools - A Review of Manufacturing RoutesDocument8 pagesPowder Metallurgy Diamond Tools - A Review of Manufacturing RoutesJan KoNo ratings yet
- Handbook of Nanophase and Nano Structured Materials 4Document344 pagesHandbook of Nanophase and Nano Structured Materials 4mohayman100% (2)
- Thermal Aspects in Metal CuttingDocument122 pagesThermal Aspects in Metal CuttingSanjay NayeeNo ratings yet