Professional Documents
Culture Documents
Epf Medical Devices Factsheet 2016
Epf Medical Devices Factsheet 2016
Uploaded by
Erik QwertOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Epf Medical Devices Factsheet 2016
Epf Medical Devices Factsheet 2016
Uploaded by
Erik QwertCopyright:
Available Formats
MEDICAL
DEVICES
WHAT IS A MEDICAL DEVICE?
An article, instrument, apparatus or This category encompasses many different devices: from common consumer products
machine that is used in the prevention, like bandages or glasses, to medical equipment used by healthcare professionals and in
diagnosis or treatment of illness or hospitals. Patients with chronic conditions use medical devices in their daily life to support
disease, or for detecting, measuring, them with the management of their conditions, sometimes in combination with medicines.
restoring, correcting or modifying the
There are also active implanted devices like stents, pacemakers or hip replacement. Some
structure or function of the body for some
medical devices are also used for telemedicine, to enable remote monitoring or
health purpose.
communication with the patients.
World Health Organisation
WHY DOES IT MATTER TO PATIENTS?
Medical devices play a key role They contribute to patients’ life Access to medical devices and to related
in diagnosis and treatment of chronic expectancy and quality of life. quality information remains crucial for
conditions, and in supporting patients patients with chronic and long term
to remain independent. conditions.
WHAT ROLE DOES THE EU PLAY IN THIS AREA?
The EU legislation aims at ensuring medical devices are safe. Medical devices have to
undergo a conformity assessment to ensure they meet standards for safety and
EU
performance. If they do, they receive a CE mark before they are placed on the
European market.
Given the diversity of devices, different rules apply according to the risk they can pose
to patients.
2016 1.
Better information to patients through an EU database on medical devices,
EUDAMED, accessible to the public and through information leaflet for
implanted patients.
2.
In 2016, The EU adopted
two Regulations, on medical
devices and in vitro diagnostics,
which will be applied 3 years.
The right for patients to directly report incidents that happen with their
medical devices allowing better prevention related to future risks.
3.
The new legislation will bring Stronger safety requirements to improve the assessment and management
important changes for patients: of risks with devices. Expert panels will give scientific advice for the
assessment of high risk devices. These expert panels are required to take
into account the views of patient organisations when drafting their
opinions.
WHAT IS PATIENT SAFETY AS A PRIORITY THROUGHOUT THE
LIFECYCLE OF THE DEVICE
EPF EPF is advocating for high quality, safe devices. There are three stages which are crucial
ADVOCATING
for patient safety: clinical investigation when the device is tested, conformity assessment
when the safety and performance of the device is assessed, and vigilance which is the
FOR?
constant monitoring of risks and incidents once the device is on the market. In our
view patients have a key role to play in each of these steps, to ensure devices meet
their needs and in reporting safety and quality issues.
TRANSPARENCY AND INFORMATION TO PATIENTS
EPF is calling for patients’ access to transparent, reliable information tailored to
patients’ needs on medical devices, to empower patients and their healthcare
professionals to make informed choices in the management of their conditions.
PATIENT INVOLVEMENT
EPF is advocating for the fundamental right of patients and their organisations to be
SAFETY meaningfully involved in key aspects of medical devices including safety and quality,
TRANSPARENCY transparency and information, and vigilance. Patients have a unique expertise as users
INVOLVEMENT
of medical devices, which needs to be better harnessed to ensure that medical devices
in the EU are safe, high quality, accessible and meet patients’ needs.
ACCESS
ACCESS
Because medical devices play a key role in prevention, diagnosis, disease
management and treatment, we call on the EU to take actions to encourage equitable
access to quality medical devices for patients according to their needs, not their
means.
Rue du Commerce 31 This infographic received funding under an operating
1000 Brussels, Belgium grant from the European Union’s Health Programme
Phone : + 32 2 280 23 34 (2014-2020).
Fax : + 32 2 231 14 47
The content of this infographic represents the views of
info@eu-patient.eu the author only and is his/her sole responsibility; it
www.eu-patient.eu cannot be considered to reflect the views of the
European Commission and/or the Consumers, Health
europeanpatientsforum/eupatient and Food Executive Agency or any other body of the
European Union. The EuropeanCommission and the
Agency do not accept any responsibility for use that
@eupatientsforum may be made of the information it contains.
www.eu-patient.eu/blog
You might also like
- Week 3Document37 pagesWeek 3Rasmina Jhoy Roque Quintana33% (3)
- Golden Shield BrochureDocument11 pagesGolden Shield BrochureRak Esh RakeshNo ratings yet
- Luise Hay Symptoms ListDocument12 pagesLuise Hay Symptoms Listmaviladin100% (1)
- Medical Product Safety and RegulationDocument77 pagesMedical Product Safety and Regulationreycardo100% (1)
- Regulation For Medical Devices in IndiaDocument5 pagesRegulation For Medical Devices in IndiaPavan kumar NaikNo ratings yet
- CorpBrochure Med ProductsDocument16 pagesCorpBrochure Med ProductsfsdfwefNo ratings yet
- Tonometers SpecificationDocument3 pagesTonometers SpecificationsamygaradaNo ratings yet
- Infographic NEW REGULATIONS-ENDocument1 pageInfographic NEW REGULATIONS-ENM J garments TprNo ratings yet
- 30 Safe Practices For Bettr HCDocument4 pages30 Safe Practices For Bettr HCkathykabommocNo ratings yet
- PatientsafetyDocument21 pagesPatientsafetytuNo ratings yet
- SFDA Telehealth-Application-Guidelines - 2023Document74 pagesSFDA Telehealth-Application-Guidelines - 2023Lackner MarceloNo ratings yet
- Efficacy and Safety of Medicines 1Document7 pagesEfficacy and Safety of Medicines 1Saida KuronboevaNo ratings yet
- Hospital CDocument162 pagesHospital CBerylsom MorrisNo ratings yet
- MDR CapabilitiesDocument20 pagesMDR CapabilitiesElexes ConsultingNo ratings yet
- Tizon, R. - Assignment#3ncm112Document3 pagesTizon, R. - Assignment#3ncm112Royce Vincent TizonNo ratings yet
- Maintenence Part 1Document69 pagesMaintenence Part 1Abeeesy AmrakeyNo ratings yet
- Medical Devices 2021Document67 pagesMedical Devices 2021Vighnesh Vikki100% (1)
- The Correlation Between Knowledge and Attitude of The Nurse Towards of Usage Personal Protective Equipment in General Hospital of PandanDocument6 pagesThe Correlation Between Knowledge and Attitude of The Nurse Towards of Usage Personal Protective Equipment in General Hospital of PandanInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- ECRI 2023 Top 10 Hazards Executive BriefDocument18 pagesECRI 2023 Top 10 Hazards Executive BriefAlejandro MartínezNo ratings yet
- GN-13-R1.1 Guidance On The Risk Classification of General Medical DevicesDocument31 pagesGN-13-R1.1 Guidance On The Risk Classification of General Medical DevicesJoresp EspinozaNo ratings yet
- Chapter 15-Reviwer NIDocument3 pagesChapter 15-Reviwer NIAbigail Ann BinalayNo ratings yet
- Key Concepts in Nursing InformaticsDocument9 pagesKey Concepts in Nursing Informaticsluchi darkNo ratings yet
- MTDocument63 pagesMTTarun GuptaNo ratings yet
- CSEIT1831304Document4 pagesCSEIT1831304YogeshNo ratings yet
- Immunisation Information Systems - Useful Tools For Monitoring Vaccination Programmes in EU/EEA Countries, 2016Document11 pagesImmunisation Information Systems - Useful Tools For Monitoring Vaccination Programmes in EU/EEA Countries, 2016Afdal FatoniNo ratings yet
- Information Technology and The SocietyDocument31 pagesInformation Technology and The SocietyJulianne Marie LacsentoNo ratings yet
- Safety Communication - EUPATI ToolboxDocument3 pagesSafety Communication - EUPATI ToolboxAnda AndreianuNo ratings yet
- Berita Rule and RegulationDocument2 pagesBerita Rule and RegulationCindy AuliaNo ratings yet
- Metrolgia Medicina 1Document5 pagesMetrolgia Medicina 1Edisson MoraNo ratings yet
- Artigo Mobile SaúdeDocument12 pagesArtigo Mobile SaúdeNathielle AlvesNo ratings yet
- @ 2 Effects of Electronic Medical Records On Patient Safety Culture: The Perspective of NursesDocument7 pages@ 2 Effects of Electronic Medical Records On Patient Safety Culture: The Perspective of NursesElizabeth CotenNo ratings yet
- IPS128Document15 pagesIPS128ElairaneNo ratings yet
- Jurnal Bedside MonitorDocument4 pagesJurnal Bedside MonitorAnonymous eCgTp1No ratings yet
- 0rder 248-WearableTechnology-WEEK5Document32 pages0rder 248-WearableTechnology-WEEK5joshua chegeNo ratings yet
- Healthcare Intelligent Architecture High LevelDocument9 pagesHealthcare Intelligent Architecture High LevelOscarNo ratings yet
- Digital Makassar - FinalDocument40 pagesDigital Makassar - FinalPutra PunjabyNo ratings yet
- 1 Chap 1 Part 1Document5 pages1 Chap 1 Part 1CAÑADA, JOHANNELYN M.No ratings yet
- Patient Safety and TechnologyDocument5 pagesPatient Safety and TechnologyAditya Toga100% (1)
- Healthcare in TechnologyDocument29 pagesHealthcare in TechnologySHRAVYA MANJUNATHNo ratings yet
- JD - General Nurse, Special ClinicDocument1 pageJD - General Nurse, Special Clinicodipo kizitoNo ratings yet
- Ijerph 20 05794 With CoverDocument22 pagesIjerph 20 05794 With CoverUmi_memey46No ratings yet
- Sensors: Smart Wearables For Cardiac Monitoring-Real-World Use Beyond Atrial FibrillationDocument25 pagesSensors: Smart Wearables For Cardiac Monitoring-Real-World Use Beyond Atrial FibrillationMohanraj TNo ratings yet
- St. Louis University Institute of Health and Biomedical SciencesDocument39 pagesSt. Louis University Institute of Health and Biomedical SciencesMind BlowerNo ratings yet
- Patient SafetyDocument7 pagesPatient SafetydeenawantsNo ratings yet
- Personal Health Monitoring System Using Arduino and Android IJERTCONV4IS22014Document5 pagesPersonal Health Monitoring System Using Arduino and Android IJERTCONV4IS22014Yasir Yasir KovanNo ratings yet
- Exploring Patient Safety Culture in Emergency DepartmentsDocument7 pagesExploring Patient Safety Culture in Emergency Departmentsmuhammad ismailNo ratings yet
- ICU Without WallDocument6 pagesICU Without WallFahmi AuliaNo ratings yet
- Internet of Medical Things: A Review of Recent Contributions Dealing With Cyber-Physical Systems in MedicineDocument13 pagesInternet of Medical Things: A Review of Recent Contributions Dealing With Cyber-Physical Systems in MedicineRafael OgawaNo ratings yet
- Special Issue 1 3Document129 pagesSpecial Issue 1 3Amit PasiNo ratings yet
- Healthcare SafetyDocument3 pagesHealthcare SafetySanjeeb KakatiNo ratings yet
- Ehealth CHN & Core Values HandoutDocument36 pagesEhealth CHN & Core Values HandoutAlfred Bueno DanganiNo ratings yet
- Effectiveness of In-Service Education On Knowledge of Nurses Regarding Prevention and Management of Sensory Alterations in Patients Admitted To ICUs of Selected Hospital, BangaloreDocument5 pagesEffectiveness of In-Service Education On Knowledge of Nurses Regarding Prevention and Management of Sensory Alterations in Patients Admitted To ICUs of Selected Hospital, BangaloreInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- WCECS2017 - pp261-264 (Remote Health Monitor)Document4 pagesWCECS2017 - pp261-264 (Remote Health Monitor)sebastian okhanigbeNo ratings yet
- Ehealth ReportDocument6 pagesEhealth ReportMaria ThereseNo ratings yet
- Io MTDocument11 pagesIo MTshuvam.bag.devNo ratings yet
- Appropriate Use Criteria For Handheld Pocket Ultrasound DevicesDocument3 pagesAppropriate Use Criteria For Handheld Pocket Ultrasound DevicesNanasNo ratings yet
- Informatics and The Healthcare IndustryDocument2 pagesInformatics and The Healthcare IndustryJaaaanNo ratings yet
- Towards International Harmonized Nomenclature For Medical DevicesDocument13 pagesTowards International Harmonized Nomenclature For Medical DevicesSachin GuptaNo ratings yet
- Medication Management Needs ICT-based Approaches IDocument8 pagesMedication Management Needs ICT-based Approaches IAnkit ChandraNo ratings yet
- Patient Safety and CommunicationDocument11 pagesPatient Safety and CommunicationJulls ApouakoneNo ratings yet
- E-Health and Storage Group 1 (NCM 113 THEORY)Document10 pagesE-Health and Storage Group 1 (NCM 113 THEORY)Ralph Elvin MacanlalayNo ratings yet
- Gunnar B. J. Andersson M.D., PH.D., Thomas W. McNeill M.D. (Auth.) - Lumbar Spine Syndromes - Evaluation and Treatment-Springer-Verlag Wien (1989)Document223 pagesGunnar B. J. Andersson M.D., PH.D., Thomas W. McNeill M.D. (Auth.) - Lumbar Spine Syndromes - Evaluation and Treatment-Springer-Verlag Wien (1989)Tatar AlexNo ratings yet
- Students Worksheet 9 English For Nursing - Corona Virus Covid-19Document5 pagesStudents Worksheet 9 English For Nursing - Corona Virus Covid-19Ihat SholihatNo ratings yet
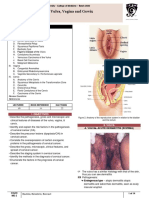
- B14PathologyL1 - Diseases of The Vulva, Vaginal, and CervixDocument14 pagesB14PathologyL1 - Diseases of The Vulva, Vaginal, and CervixBarda GulanNo ratings yet
- Steps of Repertorization - 5e097dab9ad98Document18 pagesSteps of Repertorization - 5e097dab9ad98Sowjanya JyothsnaNo ratings yet
- Clinical Microbiology and Infection: Research NoteDocument4 pagesClinical Microbiology and Infection: Research NoteDedi IrawandiNo ratings yet
- Essays On GlobalisationDocument7 pagesEssays On Globalisationd3gxt9qh100% (2)
- Ancient Greek and Greco-Roman Methods in Modern Surgical Treatment of CancerDocument3 pagesAncient Greek and Greco-Roman Methods in Modern Surgical Treatment of CancerAlexia ToumpaNo ratings yet
- Test 5 - Oral Surgery, Oral Diagnosis & RoentgenologyDocument6 pagesTest 5 - Oral Surgery, Oral Diagnosis & Roentgenologydr.jah9No ratings yet
- TYPHOIDDocument13 pagesTYPHOIDnaceyjj33No ratings yet
- 107 RLE Group 5 1F Newborn Delivery Final OutputDocument79 pages107 RLE Group 5 1F Newborn Delivery Final OutputJr CaniaNo ratings yet
- CWTS (Lesson 2) Basic Life SuportDocument19 pagesCWTS (Lesson 2) Basic Life SuportAshley Kezia LopezNo ratings yet
- Introduction To Meril InnovationDocument49 pagesIntroduction To Meril InnovationLulu MizbahNo ratings yet
- PSA Participant Manual May 2022 ApprovedDocument103 pagesPSA Participant Manual May 2022 Approvedlidyamengistu12No ratings yet
- List of Culture Media Used in Microbiology With Their UsesDocument7 pagesList of Culture Media Used in Microbiology With Their UsesImmu JiNo ratings yet
- Distonía Del Guitarrista: Tratamiento Con Reeducación SensorialDocument4 pagesDistonía Del Guitarrista: Tratamiento Con Reeducación SensorialSara M.No ratings yet
- Case Scenario 5 For StudentsDocument8 pagesCase Scenario 5 For StudentsPrince DetallaNo ratings yet
- National Ehealth StrategyDocument121 pagesNational Ehealth StrategyAnna YurevaNo ratings yet
- 3 Analyzer Antibiotic Susceptibility Testing BrochureDocument24 pages3 Analyzer Antibiotic Susceptibility Testing BrochureGlobal TominentNo ratings yet
- Mou&Sin&Exp&Gui&Cri&Car&1 STDocument642 pagesMou&Sin&Exp&Gui&Cri&Car&1 STDragutin PetrićNo ratings yet
- SOMNObalance Soft2 e 67701 enDocument72 pagesSOMNObalance Soft2 e 67701 enelahe shaahrezaeiNo ratings yet
- Auriculotherapy: Musculoskeletal & Sensory System Point LocationsDocument1 pageAuriculotherapy: Musculoskeletal & Sensory System Point LocationscocosinghNo ratings yet
- Appropriate Oral Health CareDocument21 pagesAppropriate Oral Health CareSupriya BhataraNo ratings yet
- Essay Article On Consumer Awareness Towards Food SafetyDocument4 pagesEssay Article On Consumer Awareness Towards Food SafetyMohd Faizal Bin AyobNo ratings yet
- AASLD Nomenclature Full Presentation 9.15.23 FINALDocument27 pagesAASLD Nomenclature Full Presentation 9.15.23 FINALFaisal BaigNo ratings yet
- Nasha Mukti Kendra Bhopal PDFDocument4 pagesNasha Mukti Kendra Bhopal PDFshrishuddhi1No ratings yet
- 100014Z09JM2016 Mathew PDFDocument7 pages100014Z09JM2016 Mathew PDFRameshKrishnanNo ratings yet
- Lab 3 - Modelling Time DelaysDocument30 pagesLab 3 - Modelling Time DelayslynNo ratings yet
- Sinusitis With Polyposis Presenting As Refractory Trigeminal Neuralgia Treated With Acupuncture and Chinese Herbal DecoctionDocument6 pagesSinusitis With Polyposis Presenting As Refractory Trigeminal Neuralgia Treated With Acupuncture and Chinese Herbal DecoctionCarleta StanNo ratings yet