Professional Documents
Culture Documents
States of Matter Revision
Uploaded by
diyaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
States of Matter Revision
Uploaded by
diyaCopyright:
Available Formats
Visit http://www.mathsmadeeasy.co.uk/ for more fantastic resources.
Q1) What are the three states of matter (include their state symbols)?
1
2
3
(3 marks)
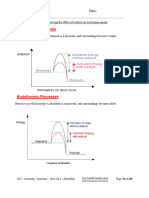
Q2) Complete the diagram to show where condensing and freezing occur.
Melting point Boiling Point
Melting Boiling
Condensing Freezing
(2 marks)
Q3) The three states of matter can be represented by a simple model. In this model, particles are
represented by small circles or spheres. Complete the model diagram.
Solid Liquid Gas
(2 marks)
Q4) What is the limitation of this model?
(1 mark)
Q5) Different substances require different amounts of energy to change state. Why is it different
amounts of energy for different substances?
(2 marks)
Maths Made Easy © Complete Tuition Ltd 2017
Visit http://www.mathsmadeeasy.co.uk/ for more fantastic resources.
Q6) Sodium chloride’s melting point is 801˚C, its boiling point is 1413˚C. On the basis of this,
complete the table.
Temperature Solid, liquid or gas
1800˚C
400˚C
805˚C
(3 marks)
Q7) Order the substances, from highest boiling to lowest boiling point according to their type of
bonds.
Highest boiling point Sodium chloride (ionic bonding)
Water (covalent)
Iron (metallic bonding)
Lowest boiling point
(3 marks)
Maths Made Easy © Complete Tuition Ltd 2017
You might also like
- GCSE Chemistry AQA OCR Edexcel. States of Matter. Answers 2Document3 pagesGCSE Chemistry AQA OCR Edexcel. States of Matter. Answers 2Cally ChewNo ratings yet
- 2.1 States of MatterDocument6 pages2.1 States of MatterGeorge TongNo ratings yet
- GCSE Science GCSE Chemistry: Moles QuestionsDocument5 pagesGCSE Science GCSE Chemistry: Moles QuestionszoeNo ratings yet
- Chemistry AQA COMBINED SCIENCE 1 (H) 2024 PREDICTEDDocument12 pagesChemistry AQA COMBINED SCIENCE 1 (H) 2024 PREDICTEDagyekumeunice76No ratings yet
- Chemistry 1 Jefado March 2020-1Document7 pagesChemistry 1 Jefado March 2020-1nassorussi9No ratings yet
- EAT227 May 2019 ExamDocument5 pagesEAT227 May 2019 ExamΚωνσταντινος ΕυρουNo ratings yet
- CBSE Class 11 Chemistry Worksheet - Some Basic Concepts of ChemistryDocument1 pageCBSE Class 11 Chemistry Worksheet - Some Basic Concepts of ChemistryNamish ManchandaNo ratings yet
- GCSE Science GCSE Chemistry: Yield and Atom Economy of Chemical Reactions. QuestionsDocument3 pagesGCSE Science GCSE Chemistry: Yield and Atom Economy of Chemical Reactions. QuestionszoeNo ratings yet
- Wa0001Document4 pagesWa0001Sajjal RanaNo ratings yet
- Particle Model of MatterDocument25 pagesParticle Model of Matterapi-422428700No ratings yet
- Kinetics QPDocument22 pagesKinetics QPdovidNo ratings yet
- Topic 2 Practice QuestionsDocument2 pagesTopic 2 Practice Questionspoyaj35718No ratings yet
- 3.2 Use of Amount of Substance On Pure Substances QP PDFDocument31 pages3.2 Use of Amount of Substance On Pure Substances QP PDFDanish FiverNo ratings yet
- Science Class Ix Periodic Test II Sample Paper 03Document4 pagesScience Class Ix Periodic Test II Sample Paper 03Ravi KumarNo ratings yet
- Kem حذف الصورDocument126 pagesKem حذف الصورturkiNo ratings yet
- A Level Chemistry Paper 2 Exam 14Document4 pagesA Level Chemistry Paper 2 Exam 14Anthony AndyNo ratings yet
- Physics AQA COMBINED SCIENCE 1 (H) 2024 PREDICTEDDocument14 pagesPhysics AQA COMBINED SCIENCE 1 (H) 2024 PREDICTEDagyekumeunice76No ratings yet
- YEAR 9 SCIENCE Exam Sample Quesyions On SHCDocument13 pagesYEAR 9 SCIENCE Exam Sample Quesyions On SHCAlayna ChattooNo ratings yet
- uploadedBrislingtonCurriculumSchool Closure ResourcesWeek 5specialist CentreYear 9Document13 pagesuploadedBrislingtonCurriculumSchool Closure ResourcesWeek 5specialist CentreYear 9Hitdifferentszaa LoveNo ratings yet
- Y6 Sample Test 1 Chemistry Answer KeyDocument3 pagesY6 Sample Test 1 Chemistry Answer KeyIrram RanaNo ratings yet
- GCSE Chemistry AQA OCR Edexcel. Structure and Bonding of Carbon Diamond and Graphite QuestionsDocument3 pagesGCSE Chemistry AQA OCR Edexcel. Structure and Bonding of Carbon Diamond and Graphite Questionssabamizban908No ratings yet
- GCSE Chemistry AQA OCR EDEXCEL. Compounds and Mixtures. QuestionsDocument4 pagesGCSE Chemistry AQA OCR EDEXCEL. Compounds and Mixtures. QuestionsRupali ParekhNo ratings yet
- Chem 9 PaperDocument2 pagesChem 9 PaperMawiz AbbasiNo ratings yet
- CHEM 1101 End of 2020 1st Sem ExamDocument5 pagesCHEM 1101 End of 2020 1st Sem ExamSaleem KholowaNo ratings yet
- 2018 Form 5 Chemistry Annual ExamDocument26 pages2018 Form 5 Chemistry Annual ExamJohnNo ratings yet
- S4 Phys pp2Document4 pagesS4 Phys pp2MASEDE JOBNo ratings yet
- Chemistry 579 February 10, 2012Document8 pagesChemistry 579 February 10, 2012Rizwan SaeedNo ratings yet
- A Level Chemistry Paper 1 Set 12marking GuideDocument17 pagesA Level Chemistry Paper 1 Set 12marking Guidebuuleivan8No ratings yet
- Matter Unit Study Guide (2) KEY: (S8P1c) (S8P1c)Document5 pagesMatter Unit Study Guide (2) KEY: (S8P1c) (S8P1c)Aayushi Jain0% (1)
- Grade 10 Paper 2Document6 pagesGrade 10 Paper 2romiifreeNo ratings yet
- Paper 1 - Higher QP Specimen PaperDocument21 pagesPaper 1 - Higher QP Specimen PaperShalini MishraNo ratings yet
- 2017-18 ICSE Class 10 Physics SQPDocument5 pages2017-18 ICSE Class 10 Physics SQPBharati GulajkarNo ratings yet
- Chemistry Class Ix Matter in Our SurroundingsDocument6 pagesChemistry Class Ix Matter in Our SurroundingstnmscharanNo ratings yet
- All YearDocument13 pagesAll YearSagar ShriNo ratings yet
- Note 28 Oct 2023Document21 pagesNote 28 Oct 2023Jihan Abou GhaddaraNo ratings yet
- S.5 Chem 2 Eot 2 2023Document6 pagesS.5 Chem 2 Eot 2 2023emakelvin040No ratings yet
- Chemistry 12 Weekly 1Document1 pageChemistry 12 Weekly 1Biswajit GhoshNo ratings yet
- Chemistry 12 Weekly 1Document1 pageChemistry 12 Weekly 1Biswajit GhoshNo ratings yet
- Aleksandra Garbera - Exam Questions ONLY On RatesDocument14 pagesAleksandra Garbera - Exam Questions ONLY On RateschemphycombNo ratings yet
- Oxford Academy Half Yearly Examination Chemistry Class: IX: Hrs MarksDocument1 pageOxford Academy Half Yearly Examination Chemistry Class: IX: Hrs Marksmahaboob kpNo ratings yet
- GCSE Chemistry AQA OCR Edexcel. Moles AnswersDocument5 pagesGCSE Chemistry AQA OCR Edexcel. Moles AnswersCally ChewNo ratings yet
- Science Class X Periodic Test II Sample Paper 01Document5 pagesScience Class X Periodic Test II Sample Paper 01hweta173No ratings yet
- s.4 Physics p2 Mock 2Document7 pagess.4 Physics p2 Mock 2amootiNo ratings yet
- March-July 2016 TEST 1 CHM420Document6 pagesMarch-July 2016 TEST 1 CHM4202022899586No ratings yet
- Class 9th t1 PaperDocument2 pagesClass 9th t1 PapermalakepayalNo ratings yet
- CHEMISTRY F6 Pre-Mock 1 July 2022Document5 pagesCHEMISTRY F6 Pre-Mock 1 July 2022Kelvin CharlesNo ratings yet
- Physics Form 1 Marking SchemeDocument12 pagesPhysics Form 1 Marking SchemeOkumu KevinsNo ratings yet
- Chapter 14 TestDocument4 pagesChapter 14 TestLeela ZeroNo ratings yet
- سلايدات 101 كيم كاملةDocument276 pagesسلايدات 101 كيم كاملةNouf AlaskarNo ratings yet
- Structure of An Atom Revision PaperDocument5 pagesStructure of An Atom Revision PaperZoe Kim ChinguwaNo ratings yet
- Reversible Reactions and Equilibria Paper1Document6 pagesReversible Reactions and Equilibria Paper1saipkNo ratings yet
- Sept-Dec 2018 TEST 1 - CHM420Document6 pagesSept-Dec 2018 TEST 1 - CHM4202022899586No ratings yet
- All India Test Series (Flash Back - Ii, Aiims) : 28.11.2016: (Time: 3 Hours) Full Marks: 200Document17 pagesAll India Test Series (Flash Back - Ii, Aiims) : 28.11.2016: (Time: 3 Hours) Full Marks: 200PriyavartNo ratings yet
- Chemistry F.SC Part 1 Test Session-2023 (Test No.1)Document1 pageChemistry F.SC Part 1 Test Session-2023 (Test No.1)Sheraz ShahNo ratings yet
- AS KineticsDocument41 pagesAS Kineticsvintu pvNo ratings yet
- UntitledDocument3 pagesUntitledLorine LowrioNo ratings yet
- P525/2 Chemistry Paper 2: Uganda Advanced Certificate of Education Page 1Document8 pagesP525/2 Chemistry Paper 2: Uganda Advanced Certificate of Education Page 1ArthurNo ratings yet
- SLG HomeworkDocument12 pagesSLG HomeworkMethyl OrangeNo ratings yet
- Section A Section A Contains Three Questions of 2-Marks Each and All Are CompulsoryDocument3 pagesSection A Section A Contains Three Questions of 2-Marks Each and All Are CompulsoryRITAN SHAIKHNo ratings yet