Professional Documents
Culture Documents
Chapter 14 Test
Uploaded by
Leela ZeroCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 14 Test
Uploaded by
Leela ZeroCopyright:
Available Formats
14 Thermal physics
OCR Physics A Exam-style questions
Refer to the Physics A data sheet for data, formulae and relationships information.
1 a Use the kinetic theory of matter to relate the properties of the solid, liquid,
and gaseous phases of a substance, to the forces and distance between its
molecules and to the motion of its molecules.
(6 marks)
b A block of aluminium at 80 ºC is fully immersed in a beaker of water at 290 K.
Describe the transfer of thermal energy between the three objects involved
and any change in temperature that may take place.
(4 marks)
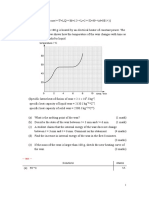
2 Gallium has a melting point of 30 ºC. Figure 1 shows how the temperature, T, of
a small mass of gallium varies when it is heated at a steady rate from
20 ºC to 40 ºC.
Figure 1
The graph shows three distinct sections labelled A, B, and C.
© Oxford University Press 2016 http://www.oxfordsecondary.co.uk/acknowledgements
This resource sheet may have been changed from the original 1
14 Thermal physics
OCR Physics A Exam-style questions
Describe and explain the features of the graph in terms of the changes which
occur to the separation and speed of the molecules and to their internal energy.
(7 marks)
3 a Define the specific heat capacity of a substance.
(1 mark)
b The specific heat capacity of a metal may be determined by using an
electrical heater embedded into a block made out of the metal. Sketch a
labelled diagram of the electrical circuit you would use.
(3 marks)
c The pitch in a football stadium is prevented from freezing by an underground
system of electrical heating cables. Prior to a match the pitch temperature
falls to 5.5 ºC to a depth of 6.0 cm. The area of the pitch is 1.3 × 104 m².
Calculate the thermal energy required to raise the temperature of the soil to
0 ºC assuming the density and specific heat capacity of the soil are
2500 kg m3 and 1600 J kg1 K1, respectively.
(3 marks)
© Oxford University Press 2016 http://www.oxfordsecondary.co.uk/acknowledgements
This resource sheet may have been changed from the original 2
14 Thermal physics
OCR Physics A Exam-style questions
4 a Derive the SI base unit for specific heat capacity.
(3 marks)
b A bottle containing 500 g of lemonade is placed in a refrigerator. The
lemonade cools from 18 °C to 4.0 °C in a time of 90 minutes.
The specific heat capacity of lemonade is 3900 J kg1 K1.
Calculate:
i the thermal energy removed from the lemonade as it cools
(2 marks)
ii the rate at which thermal energy is removed from the lemonade, in watts.
(1 mark)
5 a Define the specific latent heat of fusion of a substance.
(1 mark)
b Describe an electrical experiment to determine the specific latent heat of
vaporisation of water, L. Include in your answer:
a labelled diagram of the apparatus
a list of the measurements to be taken
an explanation of how the value of L would be determined from your
results
possible sources of uncertainty in your measurements and how these
could be reduced.
(8 marks)
© Oxford University Press 2016 http://www.oxfordsecondary.co.uk/acknowledgements
This resource sheet may have been changed from the original 3
14 Thermal physics
OCR Physics A Exam-style questions
6 a A solid substance is placed into a sealed insulated container until it
vaporises. The container is heated electrically at a constant rate until the
substance has completely vaporised. Figure 2 shows the temperature
against time graph for the entire process.
Figure 2
Use the graph to calculate for the substance:
i the ratio
specific heat capacity of the solid phase
specific heat capacity of the liquid phase
(2 marks)
ii the ratio
specific latent heat of vaporisation
specific latent heat of fusion
(2 marks)
b In an espresso coffee machine, steam at 100 ºC is passed into 250 g of milk
in order to heat it from 15 ºC to 80 ºC.
Specific heat capacity of water 4200 J kg1 K1
Specific latent heat of vaporisation of water 2.26 × 106 J kg1
Calculate the mass of steam condensed in the process.
(3 marks)
© Oxford University Press 2016 http://www.oxfordsecondary.co.uk/acknowledgements
This resource sheet may have been changed from the original 4
You might also like
- Latin OrdinaryFormMassText PDFDocument25 pagesLatin OrdinaryFormMassText PDFAlbert ArominNo ratings yet
- 50 Best PlayboyDocument25 pages50 Best PlayboyJohn Theodore Gobble III100% (3)
- Level Past Paper Questions - Hysics O P: TOPIC-8 Heat Capacity PAPER-1 Multiple ChoiceDocument3 pagesLevel Past Paper Questions - Hysics O P: TOPIC-8 Heat Capacity PAPER-1 Multiple Choiceelty Tan50% (2)
- (Norvel Hayes) How To Get Your Prayers Answered (1Document34 pages(Norvel Hayes) How To Get Your Prayers Answered (1Rosemary Hester60% (5)
- Calorimetry Class 10 Icse TESTDocument1 pageCalorimetry Class 10 Icse TESTtarun aroraNo ratings yet
- R Programming II 1 Mid QuestionbankDocument2 pagesR Programming II 1 Mid Questionbankssrkm gupta100% (1)
- Suraiya Faroqhi-Approaching Ottoman History - An Introduction To The Sources (2000)Document273 pagesSuraiya Faroqhi-Approaching Ottoman History - An Introduction To The Sources (2000)Denis Ljuljanovic100% (3)
- Triste S TropiquesDocument442 pagesTriste S TropiquesBenjamin DaughertyNo ratings yet
- Thermal Physics Long Answer QuestionsDocument5 pagesThermal Physics Long Answer QuestionsjimNo ratings yet
- Heat Capacity and Latent Heat QuestionsDocument2 pagesHeat Capacity and Latent Heat QuestionstuvvacNo ratings yet
- Other Plant Feasibility Report PDFDocument36 pagesOther Plant Feasibility Report PDFsvvsnrajuNo ratings yet
- 2013 IB Thermal Questions: (22 Marks)Document2 pages2013 IB Thermal Questions: (22 Marks)GajendraNo ratings yet
- Thermal Measurements PPQDocument4 pagesThermal Measurements PPQMichael Harrichandsingh100% (1)
- Form 3 Holiday Assignment November December 2017Document14 pagesForm 3 Holiday Assignment November December 2017maxwel oboraNo ratings yet
- SHC QUNDocument3 pagesSHC QUNYoviNo ratings yet
- (AQA) SHCandSLHDocument5 pages(AQA) SHCandSLHmarkmattesz06No ratings yet
- (Total 1 Mark) : IB Questionbank Physics 1Document5 pages(Total 1 Mark) : IB Questionbank Physics 1Irwansyah RamadhaniNo ratings yet
- YEAR 9 SCIENCE Exam Sample Quesyions On SHCDocument13 pagesYEAR 9 SCIENCE Exam Sample Quesyions On SHCAlayna ChattooNo ratings yet
- uploadedBrislingtonCurriculumSchool Closure ResourcesWeek 5specialist CentreYear 9Document13 pagesuploadedBrislingtonCurriculumSchool Closure ResourcesWeek 5specialist CentreYear 9Hitdifferentszaa LoveNo ratings yet
- 15AE33 JAN FEB 23-1 (2 Files Merged)Document2 pages15AE33 JAN FEB 23-1 (2 Files Merged)bhargavNo ratings yet
- 3 Markscheme SL Paper2Document41 pages3 Markscheme SL Paper2maddiemads562No ratings yet
- Topic 3 Past Paper: MarkschemeDocument10 pagesTopic 3 Past Paper: MarkschemeGajendra100% (1)
- Topic 3 Past Paper: MarkschemeDocument10 pagesTopic 3 Past Paper: MarkschemeLaila HassanNo ratings yet
- 3.9 Quantity of HeatDocument38 pages3.9 Quantity of Heatcicilywairimu25No ratings yet
- T - Specific Heat Capacity and Latent Heat - QuestionsDocument12 pagesT - Specific Heat Capacity and Latent Heat - QuestionsfutnitzNo ratings yet
- Thermal GuideDocument34 pagesThermal GuideMazinNo ratings yet
- 6.5 CalcDocument4 pages6.5 CalctholmesNo ratings yet
- HKDSE Phy 1A A3 - AllDocument13 pagesHKDSE Phy 1A A3 - Alljackson wongNo ratings yet
- Assignment Science Term 3Document2 pagesAssignment Science Term 3lookatthatshoeNo ratings yet
- Latent and Specific Heat - QPDocument17 pagesLatent and Specific Heat - QPCUonline OfficeNo ratings yet
- Worksheet 21Document3 pagesWorksheet 21Dariya IsmagilovaNo ratings yet
- Thermal PhysicsDocument4 pagesThermal PhysicsSavage BoiiiNo ratings yet
- 3.8 Quantity of HeatDocument18 pages3.8 Quantity of HeatKisaka GNo ratings yet
- Extension Worksheet - Topic 3, Worksheet 1Document2 pagesExtension Worksheet - Topic 3, Worksheet 1Anonymous oATq0YNo ratings yet
- 2006 Form 3 Physics Half-Yearly Exam (Dec 2006)Document4 pages2006 Form 3 Physics Half-Yearly Exam (Dec 2006)Raistlin Chan Ching KitNo ratings yet
- 2223 Level M Physics Course Questions PDFDocument76 pages2223 Level M Physics Course Questions PDFOmar HamadNo ratings yet
- Study QuestionsDocument1 pageStudy Questionskiwandaemmanuel21No ratings yet
- 6.5 BuygDocument3 pages6.5 BuygtholmesNo ratings yet
- Topic Test: Oxfordaqa International A Level PhysicsDocument15 pagesTopic Test: Oxfordaqa International A Level Physicsandhi soesiloNo ratings yet
- Thermal Practice2018Document12 pagesThermal Practice2018Trương Quốc HuyNo ratings yet
- UNIT 1 PHYS Kinetic Theory, Heat TransferDocument16 pagesUNIT 1 PHYS Kinetic Theory, Heat TransferOnaje OneNo ratings yet
- 3 6 2 1-Thermal-Energy-TransferDocument90 pages3 6 2 1-Thermal-Energy-TransferFrenzel Annie LapuzNo ratings yet
- Thermometry: T/s T/KDocument9 pagesThermometry: T/s T/KJing Yu VoonNo ratings yet
- Thermal Physics Extension QsDocument5 pagesThermal Physics Extension QsRamesh ShresthaNo ratings yet
- Chemical Engineering Thermodynamics IDocument2 pagesChemical Engineering Thermodynamics Ilata sinsinwarNo ratings yet
- Thermal Propeties of MatterDocument3 pagesThermal Propeties of MatterTanuNo ratings yet
- Thermodynamics - Top 500 Question Bank For JEE Main by MathonGoDocument20 pagesThermodynamics - Top 500 Question Bank For JEE Main by MathonGovelayudhanshree03No ratings yet
- Latent Heat QuestionsDocument2 pagesLatent Heat QuestionsSatria HalimNo ratings yet
- HKDSE Phy 1A A2 - AllDocument8 pagesHKDSE Phy 1A A2 - Alljackson wongNo ratings yet
- Dps Sts Schol: Explanetion - Ioskttsy.Nstsisal..W:M..Slst..The..Block... Snd..Esna. Hect - Jles...Document17 pagesDps Sts Schol: Explanetion - Ioskttsy.Nstsisal..W:M..Slst..The..Block... Snd..Esna. Hect - Jles...Why is LivingNo ratings yet
- Chem 201Document4 pagesChem 201dariusmakabila29No ratings yet
- Solutions MarksDocument11 pagesSolutions MarksVincent haNo ratings yet
- 3.1 - Thermal - 2020 QuestionsDocument5 pages3.1 - Thermal - 2020 QuestionsHelen TarekeNo ratings yet
- (183 Marks) : (1 Mark)Document33 pages(183 Marks) : (1 Mark)Yu SunNo ratings yet
- A2 Chemistry Assessment 1 List - REVISION RESOURCEDocument46 pagesA2 Chemistry Assessment 1 List - REVISION RESOURCEHarry BarkerNo ratings yet
- 8 Ia 6Document1 page8 Ia 6sayemaNo ratings yet
- 2021hy3p - SolutionDocument2 pages2021hy3p - SolutionEndi WongNo ratings yet
- Class Handout Unit 34bDocument3 pagesClass Handout Unit 34bKa Lok LaiNo ratings yet
- 01 Exercise Solutions e PDFDocument9 pages01 Exercise Solutions e PDFouo So方No ratings yet
- Btech Me 3 Sem Thermodynamics Dec 2017Document3 pagesBtech Me 3 Sem Thermodynamics Dec 2017Satya Priya PandeyNo ratings yet
- MYP 4&5 Physics - 1Document22 pagesMYP 4&5 Physics - 1allanngisiaNo ratings yet
- Thermal PhysicsDocument13 pagesThermal PhysicsadamfathialsalehNo ratings yet
- S4 Phys pp2Document4 pagesS4 Phys pp2MASEDE JOBNo ratings yet
- Heat Transfer: B.Tech. (Chemical Engineering) Fifth Semester (C.B.S.)Document2 pagesHeat Transfer: B.Tech. (Chemical Engineering) Fifth Semester (C.B.S.)Anurag TalwekarNo ratings yet
- Topic 3 - Cambridge - Exam Style QuestionsDocument4 pagesTopic 3 - Cambridge - Exam Style Questionsferas jehadNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- 4.production FunctionsDocument29 pages4.production FunctionsKanika BakshiNo ratings yet
- Tales of Animals in War 2023Document4 pagesTales of Animals in War 2023Brijesh Chera - Sir Isaac Brock PS (1417)No ratings yet
- Ejercicios Voz PasivaDocument3 pagesEjercicios Voz Pasivasmary_20No ratings yet
- Prospect SRSKerlaDocument23 pagesProspect SRSKerlaSonal ShahNo ratings yet
- L. Varbanova-Session V - Introduction To Arts MarketingDocument21 pagesL. Varbanova-Session V - Introduction To Arts Marketingzulejunior87No ratings yet
- Original Complaint Doc1 Redwood Dental Group vs. American Dental PartnersDocument144 pagesOriginal Complaint Doc1 Redwood Dental Group vs. American Dental PartnersDGardner242No ratings yet
- 2023 일반대학원 모집요강 (국문)Document17 pages2023 일반대학원 모집요강 (국문)Diego Andre Torres VillegasNo ratings yet
- Japanese LessonDocument12 pagesJapanese LessonVin CentNo ratings yet
- Network Database ModelDocument7 pagesNetwork Database ModelArfaqammarNo ratings yet
- AllahabadDocument2,687 pagesAllahabadRudra MishraNo ratings yet
- Rural Marketing 260214 PDFDocument282 pagesRural Marketing 260214 PDFarulsureshNo ratings yet
- Manual de Referencia OpencvDocument915 pagesManual de Referencia Opencvgaspar1983No ratings yet
- HRIS Implementation in Organizations IssDocument7 pagesHRIS Implementation in Organizations IssMary Grace BuotNo ratings yet
- Mercede ResumeDocument1 pageMercede Resumeapi-160000915No ratings yet
- Peel Health Study GuideDocument162 pagesPeel Health Study Guideemo_bmoNo ratings yet
- Maths For FinanceDocument22 pagesMaths For FinanceWonde BiruNo ratings yet
- Le Meridien IT ReportDocument34 pagesLe Meridien IT ReportSagarMishra88% (8)
- Executive Report On The Customer Experience: by Brian CantorDocument32 pagesExecutive Report On The Customer Experience: by Brian CantorMegan ShepherdNo ratings yet
- Chart AssessmentDocument8 pagesChart Assessmentcarlos perezNo ratings yet
- Did You Know That Expert Basketball Coaches BeginDocument2 pagesDid You Know That Expert Basketball Coaches BegincostasNo ratings yet
- 8th January-Morning SlotDocument39 pages8th January-Morning SlotANURAG AGGARWALNo ratings yet
- Heredity: Presented By: Pauline Grace P. CastillanoDocument22 pagesHeredity: Presented By: Pauline Grace P. CastillanoPauline CastillanoNo ratings yet