Professional Documents
Culture Documents
C72IA024EN-B - Intrinsic Viscosity of Hyaluronic Acid
Uploaded by
Martín PerezOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
C72IA024EN-B - Intrinsic Viscosity of Hyaluronic Acid
Uploaded by
Martín PerezCopyright:
Available Formats
Intrinsic Viscosity Measurements of Sodium Hyaluronate
according to EU Pharmacopoeia
Relevant for: EU Pharmacopoeia, Polymers, Zero Shear Viscosity, Intrinsic Viscosity
Sodium hyaluronate is an important raw material for various pharmaceutical and medical
applications. To ensure consistent quality and a safe application sodium hyaluronate undergoes
various tests described in the EU Pharmacopoeia. The intrinsic viscosity is one of the required
test parameters for sodium hyaluronate and can easily be measured using a Lovis 2000 M.
1.1 Tests required in EU Pharmacopoeia
The EU Pharmacopoeia is a collection of test methods
for the identification and quality control of several
pharmaceuticals/medicines. The qualitative and
quantitative composition and the tests to be carried
out on medicines, raw materials used in production,
and on the intermediates of synthesis are described.
All producers of medicines and/or substances for
pharmaceutical use must therefore apply these quality
standards in order to market their products.
Tip: Since the release of version 9.3 in July 2017, the
Rolling Ball Principle is included as part of the general
chapter 2.2.49 “Falling Ball and Automatic Rolling Ball
1 Quality Control of Sodium Hyaluronate
Viscometer Methods”.
Sodium hyaluronate is the sodium salt of hyaluronic
acid, which is naturally present in the human body. Apart from other parameters, sodium hyaluronate has
For medicinal applications, hyaluronic acid either has to be tested for its intrinsic viscosity, which has to be
to be extracted from rooster combs or is synthesized stated additionally when labeling the substance.
by bacteria under lab conditions. Sodium hyaluronate
can be classified into different grades and is widely 1.2 Lovis 2000 M/ME in pharmaceutical industry
used in several industries. Some applications are
listed in the table below. Lovis 2000 M/ME is a reliable partner in strongly
regulated fields such as the pharmaceutical industry,
Industry Application as it is compliant with 21 CFR Part 11 and a Pharma
Qualification Package can be offered.
Active pharmaceutical
Pharma Industry ingredient and pharmaceutical
excipient in various drugs In addition to dynamic and kinematic viscosity,
Injectable for joint disorders, Lovis 2000 M/ME offers an integrated polymer
Medical Industry filler in plastic surgery, gels for software for automatic calculation of polymer
wound healing
parameters such as intrinsic viscosity and molar
Cosmetics Industry
Moisturizer in creams, lotions mass.
and gels
Dietary supplement in
Food Industry combination with vitamins, food Tip: Lovis 2000 M/ME can easily be combined with
additive several other Anton Paar instruments, e.g. density
Table 1: Uses of sodium hyaluronate meters, refractometers, and pH meters, to gain more
parameters from one sample. Sample changers for
automatic filling and cleaning are an additional option.
C72IA024EN-B page 1 of 3 www.anton-paar.com
2 Samples and Sample Preparation 3 Measurements
Nine samples of sodium hyaluronate were This section describes the instrument setup and the
investigated for their intrinsic viscosity [m3/kg]. used method settings.
Sample preparation was performed as described in
the related monograph of the European 3.1 Instrumental setup
Pharmacopoeia and is described below.
For the measurement of the intrinsic viscosity [m3/kg]
2.1 Sample preparation of the hyaluronate solutions a Lovis 2000 M in flow-
through mode was used.
For the intrinsic viscosity measurements, a buffer
solution was prepared for the following dilutions of Capillary: diameter 1.59 mm /
sodium hyaluronate. borosilicate glass
Ball: diameter 1.5 mm / steel
2.1.1 Buffer solution O-rings: Viton extreme
Solution 1: 0.78 g of sodium dihydrogen phosphate
and 4.50 g of sodium chloride were diluted in 500 mL Tip: All polymer parameters derive from the “relative
of deionized water. viscosity”, which is a comparison of the runtime of the
solvent and the polymer dissolved in that solvent.
Solution 2: 1.79 g of disodium hydrogen phosphate Therefore, only the runtime is required and
and 4.50 g of sodium chloride were diluted in 500 mL Lovis 2000 M features as the perfect solution.
of deionized water.
Buffer solution: Solution 1 and 2 were mixed until a pH
value of 7.00 was reached. This buffer solution was 3.2 Method settings
filtered through a sintered glass filter prior to use.
The method Lovis Polymer (Multi Concentration) was
used for the intrinsic viscosity determination of all
2.1.2 Sodium hyaluronate dilutions
solutions. Method settings as well as special polymer
settings in the software are listed below.
Four dilutions of each sodium hyaluronate sample
were prepared from a stock solution as described
below. Measurement mode: Polymer
Temperature: 25 °C
Temperature equilibration: 20 s
Stock solution: 0.2 g of sodium hyaluronate was
Measurement cycles: 3
dissolved in 50.0 g of buffer solution by stirring the
Manual angle: Automatic
solution at 4 °C for 24 hours.
Measuring distance: Automatic
Variation coefficient: 0.3 %
The dilutions A, B, C, and D were prepared by diluting
a subsequent amount of the stock solution (for dilution
Polymer settings
A) and of dilution A (for dilution B, C, and D) with
buffer solution (Table 2). All dilutions were stirred for Calculation method: Runtime ratio
20 min and filtered through a sintered glass filter Angle count
discarding the first 10 mL. (Zero Shear Scan): 4
Use Zero Shear Scan: Yes
Concentration unit: kg/m3
Dilution Stock solution Dilution A
Amount buffer Calculation method: Martin
solution
Dilution A 5.0 g - 100.0 g
Tip: Lovis has an integrated Zero Shear Scan
Dilution B - 30.0 g 10.0 g
function. This means that a solution is automatically
Dilution C - 20.0 g 20.0 g measured at various angles and the result is
Dilution D - 10.0 g 30.0 g extrapolated to a hypothetical zero shear rate.
Table 2: Preparation of the sodium hyaluronate solutions A, B, C,
and D
C72IA024EN-B page 2 of 3 www.anton-paar.com
3.3 Results 4 Conclusion
In the following the results of the intrinsic viscosity
The Lovis 2000 M is well suited for measurements in
measurements using the Lovis 2000 M are presented.
the pharmaceutical industry. The measuring principle
is an accepted method in the EU Pharmacopoeia and
Four dilutions of each sample (1 to 9) were measured, US Pharmacopeia and the instrument delivers precise
from which the intrinsic viscosity [m3/kg] was results from very small sample volumes. Combination
calculated according to Martin (Table 3) with sample changers for automatic filling and
cleaning can facilitate users' lab routine.
3
Sample Intrinsic viscosity [m /kg]
Sodium hyaluronate 1 1.484
Sodium hyaluronate 2 2.558
Sodium hyaluronate 3 0.830
Sodium hyaluronate 4 1.538
Sodium hyaluronate 5 1.536
Sodium hyaluronate 6 2.539
Sodium hyaluronate 7 1.518
Sodium hyaluronate 8 2.278
Sodium hyaluronate 9 2.138
3
Table 3: Intrinsic viscosity [m /kg] of nine sodium hyaluronate
samples
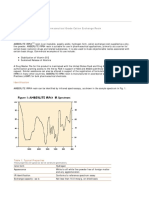
Table 4 shows the excellent repeatability of seven Figure 1: Lovis 2000 M in flow-through filling mode
subsequent measurements of the same sample.
3
Sample Intrinsic viscosity [m /kg]
Sodium hyaluronate 1 (a) 1.484
5 References
Sodium hyaluronate 1 (b) 1.529
Sodium hyaluronate 1 (c) 1.523
1. European Pharmacopoeia 9.0, Monograph "Sodium
Sodium hyaluronate 1 (d) 1.523 Hyaluronate"
Sodium hyaluronate 1 (e) 1.553
Sodium hyaluronate 1 (f) 1.516 Acknowledgement
Sodium hyaluronate 1 (g) 1.515 Special thanks to Mr. Jisa kamil, Ph.D., from Contipro
3 Pharma a.s., who performed the measurements
Mean value [m /kg] 1.52
3
Standard deviation [m /kg] 0.02
Contact Anton Paar GmbH
RSD [%] 1.35
Table 4: Repeatability in [%] of the subsequent measurement of
sodium hyaluronate sample 1 support-visco@anton-paar.com
C72IA024EN-B page 3 of 3 www.anton-paar.com
You might also like
- Pharmaceutical Impurities: An Overview: August 2010Document10 pagesPharmaceutical Impurities: An Overview: August 2010GarimaNo ratings yet
- OctaveDocument2 pagesOctaveDavid CovatzinNo ratings yet
- New Features of IP 20107592310671Document7 pagesNew Features of IP 20107592310671Banu PetiwalaNo ratings yet
- 5.4. Residual Solvents 50400eDocument8 pages5.4. Residual Solvents 50400eGiang NguyenNo ratings yet
- Malathion 2013Document80 pagesMalathion 2013Arifin R HidayatNo ratings yet
- MethomylDocument25 pagesMethomylNugroho HartonoNo ratings yet
- Eph-Admisjosnsoa, 3883n, Chem 214Document10 pagesEph-Admisjosnsoa, 3883n, Chem 214Rajat ChauhanNo ratings yet
- PKI - 2011 - AN - The Determination of Minerals in Vitamins by FAAS With PinAAcle 900Document3 pagesPKI - 2011 - AN - The Determination of Minerals in Vitamins by FAAS With PinAAcle 900Adelitza StrubingerNo ratings yet
- Impurity ProfileDocument17 pagesImpurity ProfileNishit SuvaNo ratings yet
- ShowPDF Paper - AspxDocument6 pagesShowPDF Paper - AspxHimanshu PatelNo ratings yet
- Bai Giang SX Thuoc Long - T Thanh - 2022Document54 pagesBai Giang SX Thuoc Long - T Thanh - 2022Hoang Duong Quang maiNo ratings yet
- Excipients Attributes Crucial For Parenteral PreparationsDocument11 pagesExcipients Attributes Crucial For Parenteral PreparationsBlueSagaNo ratings yet
- EVALUATION OF CERTAIN Food Aditives PDFDocument186 pagesEVALUATION OF CERTAIN Food Aditives PDFDiego Bermudez NaranjoNo ratings yet
- Bp2023 - Volume VDocument1,223 pagesBp2023 - Volume VGIGI ROCIO HARO MARIÑOS100% (2)
- HPLCDeterminationof Somefrequentlyused Parabensin SunscreensDocument7 pagesHPLCDeterminationof Somefrequentlyused Parabensin SunscreensANGIE CATALINA GALINDO GALINDONo ratings yet
- Dimethoate Eval Specs WHO June 2012Document33 pagesDimethoate Eval Specs WHO June 2012MartuaHaojahanSaragihSidabutarNo ratings yet
- 2 USP - OSD Quality TestsDocument6 pages2 USP - OSD Quality TestsSpectre SpectreNo ratings yet
- Bp2023 - Volume IVDocument964 pagesBp2023 - Volume IVGIGI ROCIO HARO MARIÑOS100% (1)
- Foamquaity 11Document18 pagesFoamquaity 11MuthumaniNo ratings yet
- Amberlite Irp64Document5 pagesAmberlite Irp64NoemiNo ratings yet
- Topical and TransdermalDocument4 pagesTopical and Transdermalshamsi009No ratings yet
- WHO On PropoxurDocument25 pagesWHO On Propoxurgilang kusumasariNo ratings yet
- Liquid Liquid Extraction Separating The MixturesDocument8 pagesLiquid Liquid Extraction Separating The MixturesMuhammad ShahbazNo ratings yet
- Review of The Oral Toxicity of Polyvinyl Alcohol (PVA)Document8 pagesReview of The Oral Toxicity of Polyvinyl Alcohol (PVA)Mădălina VargaNo ratings yet
- The Autopol IV Automatic Polarimeter: For Research, Analysis and ControlDocument8 pagesThe Autopol IV Automatic Polarimeter: For Research, Analysis and ControlAgilentechNo ratings yet
- Risk Assessment Single-Use White Paper en MRK March 2017 LowDocument4 pagesRisk Assessment Single-Use White Paper en MRK March 2017 LowRui PiresNo ratings yet
- Novel Soft Contact Lens Disinfection With Sodium Chlorite - Preservative-FreeDocument3 pagesNovel Soft Contact Lens Disinfection With Sodium Chlorite - Preservative-FreefolitasNo ratings yet
- Intranasal Drug Delivery - Drug Development Considerations (PDFDrive)Document28 pagesIntranasal Drug Delivery - Drug Development Considerations (PDFDrive)HarshaNo ratings yet
- Bp2023 - Volume IIDocument1,364 pagesBp2023 - Volume IIGIGI ROCIO HARO MARIÑOS100% (3)
- Review Article About The Development and Trends in Preservative Legislation and Safe Alternatives For The Future Verstatil DermosoftDocument5 pagesReview Article About The Development and Trends in Preservative Legislation and Safe Alternatives For The Future Verstatil DermosoftKebunLasti KurniaNo ratings yet
- Celulosa MicrocristalinaDocument4 pagesCelulosa MicrocristalinaEva Maria RamosNo ratings yet
- Avicel PH 101 & PH 102 - Microcrystalline Cellulosa PH 101 & PH 102 (p.129-133) 158-162Document5 pagesAvicel PH 101 & PH 102 - Microcrystalline Cellulosa PH 101 & PH 102 (p.129-133) 158-162Marsha Fendria PrastikaNo ratings yet
- Evaluation of Cleanroom Disinfectants: Standard Guide ForDocument5 pagesEvaluation of Cleanroom Disinfectants: Standard Guide ForKafka SetiadiNo ratings yet
- MoleculesDocument13 pagesMoleculesMuhammad Ali SyedNo ratings yet
- 01piroxicamemulgel PDFDocument14 pages01piroxicamemulgel PDFNymas PutriNo ratings yet
- Pharcal 10 12Document11 pagesPharcal 10 12The Dededo NativeNo ratings yet
- Fao Specifications and Evaluations For Agricultural PesticidesDocument44 pagesFao Specifications and Evaluations For Agricultural Pesticidesivanjose09No ratings yet
- Aatcc DetergentDocument2 pagesAatcc DetergentOliver Everett Espino100% (1)
- Spectrophotometric Method Development and ValidatiDocument7 pagesSpectrophotometric Method Development and ValidatiEVELYN SOLANHS ACERO RODRIGUEZNo ratings yet
- Excipient Qualification GuideDocument66 pagesExcipient Qualification Guidevbads67% (3)
- Chemical Analysis of Sodium Chloride: Standard Test Methods ForDocument7 pagesChemical Analysis of Sodium Chloride: Standard Test Methods ForDavid PachonNo ratings yet
- Saponification Number of Petroleum Products: Standard Test Methods ForDocument9 pagesSaponification Number of Petroleum Products: Standard Test Methods ForLuigi MazzuccoNo ratings yet
- ASTM E291 - 09 - Standard Test Methods Forchemical Analysis of Caustic Soda and Caustic Potash (Sodium Hydroxide and Potassium Hydroxide) 1Document15 pagesASTM E291 - 09 - Standard Test Methods Forchemical Analysis of Caustic Soda and Caustic Potash (Sodium Hydroxide and Potassium Hydroxide) 1Boby WongNo ratings yet
- Brochure Cleaning Emeia Product CatalogDocument40 pagesBrochure Cleaning Emeia Product Catalogpkh29No ratings yet
- Cosmetics Testing - Stability Testing. Physico-ChemicalDocument3 pagesCosmetics Testing - Stability Testing. Physico-ChemicalSérgio SantosNo ratings yet
- Poster B12Document1 pagePoster B12Vikas GoyalNo ratings yet
- Sgs Crs Eu Cosmetic Product Safety Report enDocument4 pagesSgs Crs Eu Cosmetic Product Safety Report enAbhishek SharmaNo ratings yet
- Standard Operating Procedure Filter Impregnated PaperDocument17 pagesStandard Operating Procedure Filter Impregnated Paperharis pratamaNo ratings yet
- TDS Koralone HP 150Document8 pagesTDS Koralone HP 150Quoc ThanhNo ratings yet
- Astm E70Document6 pagesAstm E70Rubén Darío Vera LópezNo ratings yet
- Formulation and Evaluation of Nimodipine Tablet by Liquisolid TechniqueDocument7 pagesFormulation and Evaluation of Nimodipine Tablet by Liquisolid TechniqueEditor IJTSRDNo ratings yet
- Controlling The Physical Properties and Performance of Semi-Solid Formulations Through Excipient SelectionDocument6 pagesControlling The Physical Properties and Performance of Semi-Solid Formulations Through Excipient SelectionAhmed Osama ShalashNo ratings yet
- Measurement of Remaining Primary Antioxidant Content in In-Service Industrial Lubricating Oils by Linear Sweep VoltammetryDocument14 pagesMeasurement of Remaining Primary Antioxidant Content in In-Service Industrial Lubricating Oils by Linear Sweep VoltammetryJuan F AlvarezNo ratings yet
- Initial PH (I-Ph) - Value of Petroleum Products: Standard Test Method ForDocument5 pagesInitial PH (I-Ph) - Value of Petroleum Products: Standard Test Method ForasmaNo ratings yet
- Code of Practice IER 20191017Document17 pagesCode of Practice IER 20191017RVaranasiNo ratings yet
- THEORANGEBOOKDocument6 pagesTHEORANGEBOOKMashiko DakhundaridzeNo ratings yet
- Absorben DiaionDocument37 pagesAbsorben DiaionRifqi AkmalNo ratings yet
- Acidity in Acrylonitrile: Standard Test Method ForDocument3 pagesAcidity in Acrylonitrile: Standard Test Method ForEric GozzerNo ratings yet
- Biosurfactants: A Boon to Healthcare, Agriculture & Environmental SustainabilityFrom EverandBiosurfactants: A Boon to Healthcare, Agriculture & Environmental SustainabilityNo ratings yet
- C72IB008EN A ShortInstr Pressurized BottleDocument2 pagesC72IB008EN A ShortInstr Pressurized BottleMartín PerezNo ratings yet
- Brochure DMA M Series ScreenDocument9 pagesBrochure DMA M Series ScreenMartín PerezNo ratings yet
- C72IB012EN-C - Lovis Polymer Measurement Short InstructionDocument20 pagesC72IB012EN-C - Lovis Polymer Measurement Short InstructionMartín PerezNo ratings yet
- Sensors 442370 DoneDocument12 pagesSensors 442370 DoneMartín PerezNo ratings yet
- XDLIP027EN-B Brochure DensityRedefined ScreenDocument7 pagesXDLIP027EN-B Brochure DensityRedefined ScreenMartín PerezNo ratings yet
- Intrinsic Viscosity Determination of High Molecular Weight Biopolymers by Different Plot Methods Masuelli 2018Document13 pagesIntrinsic Viscosity Determination of High Molecular Weight Biopolymers by Different Plot Methods Masuelli 2018Martín PerezNo ratings yet
- C92IA010EN-B Molar Mass CalculationDocument3 pagesC92IA010EN-B Molar Mass CalculationMartín PerezNo ratings yet
- C72IA030EN-B Viscosity PH Nasal Spray DropsDocument3 pagesC72IA030EN-B Viscosity PH Nasal Spray DropsMartín PerezNo ratings yet
- C72IA017EN-A Single-Point Polymer CalculationsDocument6 pagesC72IA017EN-A Single-Point Polymer CalculationsMartín PerezNo ratings yet
- C72IA025EN-B Viscosity of Whole BloodDocument3 pagesC72IA025EN-B Viscosity of Whole BloodMartín PerezNo ratings yet
- C72IA027EN-B - Viscosity of Battery ElectrolytesDocument4 pagesC72IA027EN-B - Viscosity of Battery ElectrolytesMartín PerezNo ratings yet
- C72IA001EN-C Application Report ChitosanDocument4 pagesC72IA001EN-C Application Report ChitosanMartín PerezNo ratings yet
- Bohr Model ActivityDocument2 pagesBohr Model ActivityAnonymous 7QjNuvoCpINo ratings yet
- Synthesis of Aspirin From Salicylic Acid and Acetic AnhydrideDocument6 pagesSynthesis of Aspirin From Salicylic Acid and Acetic AnhydrideChristine71% (7)
- Activity 3 Osmosis: ObjectivesDocument2 pagesActivity 3 Osmosis: ObjectivesMitchelle OclaritNo ratings yet
- Leader in Superabrasive Finishing Systems: Pad Applications We Offer A Full Line of Hyprez® ProductsDocument2 pagesLeader in Superabrasive Finishing Systems: Pad Applications We Offer A Full Line of Hyprez® Productsarmin heidariNo ratings yet
- Science 9 2nd Quiz #02 Organic CompoundsDocument2 pagesScience 9 2nd Quiz #02 Organic Compoundsryan bersamin100% (1)
- Astm-Asme Material ListDocument34 pagesAstm-Asme Material Listhakseo50% (2)
- CNS SIMDIS Product BrochureDocument4 pagesCNS SIMDIS Product BrochureAhmed TaherNo ratings yet
- F.Y.B.Sc. Chemistry Syllabus PDFDocument26 pagesF.Y.B.Sc. Chemistry Syllabus PDFBhushan jadhavNo ratings yet
- Metallographic Interpretation of Steel Forging DefectsDocument3 pagesMetallographic Interpretation of Steel Forging DefectsSinan ChenNo ratings yet
- Fyrewashf 22021Document2 pagesFyrewashf 22021noble.nwalozieNo ratings yet
- Monarch's Waterproofing PDFDocument31 pagesMonarch's Waterproofing PDFMonarch DigitalNo ratings yet
- Suppino Impregnation MethodDocument15 pagesSuppino Impregnation Methodioanaandra5690No ratings yet
- Synthesis of Single Phase Aragonite Precipitated Calcium Carbonate in Ca (Oh) - Na Co - Naoh Reaction SystemDocument5 pagesSynthesis of Single Phase Aragonite Precipitated Calcium Carbonate in Ca (Oh) - Na Co - Naoh Reaction Systemerliana nduruNo ratings yet
- 0 Corrosion Resistance Ref TableDocument1 page0 Corrosion Resistance Ref TablepvaldezmtzNo ratings yet
- Anexo Norma HolandesaDocument13 pagesAnexo Norma HolandesaThePomboNo ratings yet
- IJER Casein NehaTripathiDocument4 pagesIJER Casein NehaTripathiSamyuktha GunasekaranNo ratings yet
- FyQ Tema 3Document14 pagesFyQ Tema 3Danyel Rodriguez RomeraNo ratings yet
- Pharmaceutical Chemistry Chapter 2 P. AnalysisDocument22 pagesPharmaceutical Chemistry Chapter 2 P. AnalysisAaQib Ali RaZaNo ratings yet
- Ni (CO) 4 PDFDocument8 pagesNi (CO) 4 PDFAthira VishnuNo ratings yet
- 6-Aircraft Painting and Finishing - TextDocument3 pages6-Aircraft Painting and Finishing - TextGian DumasNo ratings yet
- Motor CleaningMethods Bishop 0620Document21 pagesMotor CleaningMethods Bishop 0620Rolando LoayzaNo ratings yet
- CHE112P Lecture Recycle - BypassDocument22 pagesCHE112P Lecture Recycle - BypassYzeNo ratings yet
- Amberlite IRN 160 LDocument2 pagesAmberlite IRN 160 LJayanath Nuwan SameeraNo ratings yet
- Ba Nr. 125 - 2016 REV - CHIM. (Bucharest) 67 No. 8 2016 MarianaPopeDocument3 pagesBa Nr. 125 - 2016 REV - CHIM. (Bucharest) 67 No. 8 2016 MarianaPopeIONITA GABRIELNo ratings yet
- Toilet Bowl Cleaner With BleachDocument1 pageToilet Bowl Cleaner With BleachWellington Silva100% (1)
- Chemistry Edexcel RevisionDocument3 pagesChemistry Edexcel RevisionchogoNo ratings yet
- 10 - Mineral GroupsDocument14 pages10 - Mineral Groupsmoza100% (1)
- Label DataDocument2 pagesLabel Datafjkhan1No ratings yet
- Mild and Efficient Cyclization Reaction of 2-Ethynylaniline Derivatives To Indoles in Aqueous MediumDocument7 pagesMild and Efficient Cyclization Reaction of 2-Ethynylaniline Derivatives To Indoles in Aqueous MediumSaurav BhattacharjeeNo ratings yet
- Star Anise (Illicium Verum) : Chemical Compounds, Antiviral Properties, and Clinical RelevanceDocument20 pagesStar Anise (Illicium Verum) : Chemical Compounds, Antiviral Properties, and Clinical RelevanceMARCIO RENATO MARQUES GAERTNERNo ratings yet