Professional Documents
Culture Documents
New photochromic cyclopentenone-fused naphthopyran
Uploaded by
Sky SousaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
New photochromic cyclopentenone-fused naphthopyran
Uploaded by
Sky SousaCopyright:
Available Formats
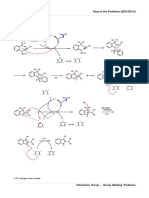
SYNTHESIS OF NEW PHOTOCHROMIC
CYCLOPENTENONE-FUSED NAPHTHO[1,2-b]PYRAN
OHO F F
H
O O O O
t-B O K
u
F O O O
H
F
O O
O
Céu M. Sousa, Luis M. Carvalho, Paulo J. Coelho
O F
P h
O O O
O 4 P h 3
%
7 6 3 %
Centro de Química, Univ. de Trás-os-Montes e Alto Douro, 5001-911 Vila Real, Portugal. pcoelho@utad.pt
INTRODUCTION SYNTHESIS
The continuous irradiation of naphtho[1,2-b]pyrans with UV light The reaction of naphthopyran-4-one 1 with the silyl enol ether 2 in the
produces two coloured photoisomers, named TC and TT, that in the absence presence of TiCl4 gave naphthopyran 3 with 75% yield. Basic hydrolysis of
of light return to the original colourless state. These compounds have met ester 3 gave the acid 4 that was subsequently converted to the respective
considerable success in the production of variable-transmission optical acyl chloride and cyclised under TiCl4 catalysis affording the
materials, namely photochromic plastic ophthalmic lenses, which darken in cyclopentenone-fused naphtho[1,2-b]pyran 5 with 48% yield.
the sunlight. Ph Ph
Ph Ph Ph Ph
Ph OSiMe3 O
Ph Ph O O
2
OCH3
O O Ph O Ph O NaOH
UV EtOH
TiCl4 , CH2Cl2 H3CO O
+ Ph
3
HO O
∆, vis 1 75% 76%
4
1) SOCl2
Closed Form 48 %
TC TT 2) TiCl4
Colourless Two coloured photoisomers
Ph Ph
O
Although exhibiting similar absorption spectra, these photoisomers show
very different thermal stabilities. While the TC isomer rapidly returns to the 5
uncoloured closed form, the TT isomer is thermally more stable and shows,
RESULTS O
2 3
normally, fading rates 10 -10 times slower. This isomer is thus responsible
for the persistence of a residual colour for several minutes/hours after the Continuous UV light irradiation (150 W ozone free Xe lamp) of a
-3
removal of the light source. This can be a serious limitation for organic uncoloured 10 M toluene solution of naphthopyran 5, at 20ºC, lead to the
ophthalmic lenses applications. development of a pale yellow colouration with a maximal absorption at 440
nm. When the UV irradiation was turned off, the absorbance decreased
1.4
Dark rapidely reaching the initial value in 1.5 min. The fading kinetic is mono-
1.2
exponential with a half-time life of 10 s which is consistent with the
1.0 formation of only one coloured photoisomer.
Although the photochromic behaviour of this compound was
Absorbance
0.8
0.6 reproducible, the maximum absorbance attained under UV continuous
0.4 irradiation decreased along with the increase of the number of
0.2 UV ligth colouration/decolouration cycles. This points to the degradation of the
compound under the experimental conditions.
0.0
0 20 40 60 80
Time (min)
0.006
OBJECTIVE
0.005
Dark
In order to prevent the formation of the long-lived TT-photoisomer we have
0.004
envisaged the preparation of some new naphthopyrans possessing an alkyl
Absorbance
bridge between the pyran double bond and the naphthalenic moiety. This 0.003
bridge can block the rotation that occurs after the pyran ring opening avoiding
0.002
the formation of the TT-isomer.
0.001
UV
Ph
Ph Ph
0.000
O O Ph
0 5 10 15 20 25
UV
Only one coloured Time (min)
photoisomer
∆, vis
Ph
Ph Ph
O O Ph
Acknowledgements: To FCT (Portugal's Foundation for Science and Technology) UV
for financial support through project PTDC/QUI/66012/2006.
∆
O O
5
Only one coloured photoisomer
You might also like
- Enzymes Dependent on Pyridoxal Phosphate and Other Carbonyl Compounds as Cofactors: Proceedings of the 8th International Symposium on Vitamin B6 and Carbonyl Catalysis, Held in Osaka, Japan, October 15 -19, 1990From EverandEnzymes Dependent on Pyridoxal Phosphate and Other Carbonyl Compounds as Cofactors: Proceedings of the 8th International Symposium on Vitamin B6 and Carbonyl Catalysis, Held in Osaka, Japan, October 15 -19, 1990T. FukuiNo ratings yet
- Physical Organic Chemistry—Ii: Specially Invited Lectures Presented at the Second IUPAC Conference on Physical Organic Chemistry Held at Noordwijkerhout, Netherlands, 29 April–2 May 1974From EverandPhysical Organic Chemistry—Ii: Specially Invited Lectures Presented at the Second IUPAC Conference on Physical Organic Chemistry Held at Noordwijkerhout, Netherlands, 29 April–2 May 1974Th. J. De BoerNo ratings yet
- Porphyrins PDFDocument17 pagesPorphyrins PDFPk JaatNo ratings yet
- Proline PDFDocument52 pagesProline PDFRathinNo ratings yet
- LC-MS Compatible Stability Indicating RP-UPLC Method For The Estimation of Ester Prodrug of Mycophenolic Acid in Injection FormulationDocument10 pagesLC-MS Compatible Stability Indicating RP-UPLC Method For The Estimation of Ester Prodrug of Mycophenolic Acid in Injection FormulationRatnakaram Venkata NadhNo ratings yet
- MSM Project-2Document14 pagesMSM Project-2Shamim AkterNo ratings yet
- A Metal-Free Bifunctional Electrocatalyst For Oxygen Reduction and Oxygen Evolution ReactionsDocument9 pagesA Metal-Free Bifunctional Electrocatalyst For Oxygen Reduction and Oxygen Evolution Reactionssatyajit beheraNo ratings yet
- Efficient synthesis of a bacterial translocase MraY inhibitorDocument4 pagesEfficient synthesis of a bacterial translocase MraY inhibitorVincent GeruszNo ratings yet
- A CONVENIENT ROUTE TO NEW PYRROLO (1,2-c) PYRIMIDONEDocument10 pagesA CONVENIENT ROUTE TO NEW PYRROLO (1,2-c) PYRIMIDONEĐặngChíHiềnVNNo ratings yet
- Ecteinascidin 743 (080414-TKGP) synthesis highlightsDocument3 pagesEcteinascidin 743 (080414-TKGP) synthesis highlightsPercival GalahadNo ratings yet
- Artigo 4Document17 pagesArtigo 4ELISANGELA SILVANo ratings yet
- Bao Cao Noi Ho Hap An NguyenDocument6 pagesBao Cao Noi Ho Hap An NguyenÂn NguyễnNo ratings yet
- Quino LoneDocument28 pagesQuino LoneanggaririnNo ratings yet
- BIO 110 Part 14: DNA ReplicationDocument37 pagesBIO 110 Part 14: DNA Replicationsunny660No ratings yet
- Photoexcited Molecules of Pteridine DerivativesDocument6 pagesPhotoexcited Molecules of Pteridine DerivativesDr. Amrit MitraNo ratings yet
- P, S, C C C o Nptel PDFDocument56 pagesP, S, C C C o Nptel PDFRathinNo ratings yet
- Fluorination of Organic Com Pounds: Reaction TypesDocument7 pagesFluorination of Organic Com Pounds: Reaction TypesSankar AdhikariNo ratings yet
- Chemistry Amino Acids PDFDocument56 pagesChemistry Amino Acids PDFChitrasen GuptaNo ratings yet
- Lec6 PhosphineCarbeneLigandsDocument22 pagesLec6 PhosphineCarbeneLigandsashishNo ratings yet
- Lecture # 29-30 Chapter 10A DNA Replication: Ec Exam Ii Mastering CH 10Document15 pagesLecture # 29-30 Chapter 10A DNA Replication: Ec Exam Ii Mastering CH 10kjhfNo ratings yet
- Rajesh Sir Protecting GPDocument6 pagesRajesh Sir Protecting GPVishalNo ratings yet
- Lecture 6Document18 pagesLecture 6Jason Allen TibonNo ratings yet
- Favorskii R ClaydenDocument3 pagesFavorskii R Claydenarchi KumarNo ratings yet
- Selective C-H Tri Uoromethoxylation of (Hetero) Arenes As Limiting ReagentDocument9 pagesSelective C-H Tri Uoromethoxylation of (Hetero) Arenes As Limiting ReagentJoha Castillo JaramilloNo ratings yet
- 31p NMRDocument17 pages31p NMRperulageaNo ratings yet
- Kher 2014Document4 pagesKher 2014Chinar PatelNo ratings yet
- 2006 CHM6108 L7L8 HandoutDocument17 pages2006 CHM6108 L7L8 Handoutaidar.seralinNo ratings yet
- Fenil Propanoid Kba 2022 FixDocument44 pagesFenil Propanoid Kba 2022 FixDevi ayuNo ratings yet
- An Aldol Condensation To Synthesize ChalconesDocument6 pagesAn Aldol Condensation To Synthesize ChalconesAssyakurNo ratings yet
- 5.36 Biochemistry Laboratory: Mit OpencoursewareDocument11 pages5.36 Biochemistry Laboratory: Mit OpencoursewareNeenu RajputNo ratings yet
- Biskra Sayad RayeneDocument1 pageBiskra Sayad Rayenerayene sayadNo ratings yet
- Photochemistry: Benzopinacol SynthesisDocument3 pagesPhotochemistry: Benzopinacol SynthesisJulius Victorius Aragon SaluriaNo ratings yet
- Ijcb 44B (10) 2114-2119Document6 pagesIjcb 44B (10) 2114-2119Shaikh SalmanNo ratings yet
- Biosynthesis of Natural Products Derived From Shikimic Acid 4.1. Phenyl-Propanoid Natural Products (C - C)Document22 pagesBiosynthesis of Natural Products Derived From Shikimic Acid 4.1. Phenyl-Propanoid Natural Products (C - C)Preeti YadavNo ratings yet
- C-Glycosylflavonoids Identification, Bioactivity and SynthesisDocument22 pagesC-Glycosylflavonoids Identification, Bioactivity and SynthesisJemiNo ratings yet
- Stereoselective Synthesis of 4-Pyranone Heterocycles via Asymmetric Aldol/Vinylogous Aldol CascadeDocument11 pagesStereoselective Synthesis of 4-Pyranone Heterocycles via Asymmetric Aldol/Vinylogous Aldol CascadeNathan Ray AlimNo ratings yet
- Matheus Síntese de Produto Natural 439Document4 pagesMatheus Síntese de Produto Natural 439MATHEUS PHILYPI ALVES VAZNo ratings yet
- On The Electrophilic Reactivities of Acarbonyl Heterocylces and ArenesDocument7 pagesOn The Electrophilic Reactivities of Acarbonyl Heterocylces and ArenesFinn NelsonNo ratings yet
- Keys To The Problems (2021/02/13) : Blue LEDDocument4 pagesKeys To The Problems (2021/02/13) : Blue LEDHuân TrầnNo ratings yet
- Lecture5 OC PDFDocument39 pagesLecture5 OC PDFAnil KumarNo ratings yet
- Novel Synthesis of Benzofuran - and Indol-2-Yl-Methanamine Derivatives PDFDocument6 pagesNovel Synthesis of Benzofuran - and Indol-2-Yl-Methanamine Derivatives PDFMiguelAlejandroMantaChavezNo ratings yet
- Functional Group InterconversionDocument4 pagesFunctional Group InterconversionPG ChemistryNo ratings yet
- El Khoury 2010Document33 pagesEl Khoury 2010Ngọc Phương Vy NguyễnNo ratings yet
- A P V Platform For Oligonucleotide SynthesisDocument12 pagesA P V Platform For Oligonucleotide Synthesismylove_withyou2001No ratings yet
- Martin 1975Document10 pagesMartin 1975Danilo FerreiraNo ratings yet
- C=C Bond Formation Reaction OverviewDocument12 pagesC=C Bond Formation Reaction Overviewaggelisgeorge8546No ratings yet
- Wittig ReactionDocument4 pagesWittig Reactionnur cahya ningrumNo ratings yet
- Regulatory Enzyme MechanismsDocument15 pagesRegulatory Enzyme MechanismsoczhinviaNo ratings yet
- Practical FCHG412 Prodrugs: Module Outcomes For The PracticalDocument14 pagesPractical FCHG412 Prodrugs: Module Outcomes For The PracticalGreg RalphNo ratings yet
- CHY8821 Assignment2 MACarroll 1718Document2 pagesCHY8821 Assignment2 MACarroll 1718Marco CignaNo ratings yet
- Yushchenko Et Al - 2021 - Glyphosate, Methods of SynthesisDocument11 pagesYushchenko Et Al - 2021 - Glyphosate, Methods of SynthesisJoana AlvesNo ratings yet
- Oxidation of Benzoin Into Benzil (N°39) : Tatiana Pachova BSC 2, Chemistry Assistant: Chandan Dey Sciences Ii - Lab. ADocument4 pagesOxidation of Benzoin Into Benzil (N°39) : Tatiana Pachova BSC 2, Chemistry Assistant: Chandan Dey Sciences Ii - Lab. ARabiaNo ratings yet
- Tetrahedron: Kamaljit Singh, Divya Arora, Jan BalzariniDocument6 pagesTetrahedron: Kamaljit Singh, Divya Arora, Jan BalzariniÁngel Demian Granados HernándezNo ratings yet
- 19 Monomer Card Game PDFDocument7 pages19 Monomer Card Game PDFatiqah90No ratings yet
- Lectures P Block Elements 3 HypervalencyDocument26 pagesLectures P Block Elements 3 HypervalencyKartik RanaNo ratings yet
- Chapter 4 ContiuedDocument24 pagesChapter 4 ContiuedumarNo ratings yet
- FDA experience with migration of perfluorochemicals from food packagingDocument40 pagesFDA experience with migration of perfluorochemicals from food packagingSrujanKumarNo ratings yet
- Camostat Mesilate : Treatment of Chronic PancreatitisDocument2 pagesCamostat Mesilate : Treatment of Chronic Pancreatitissibadatta senapatiNo ratings yet
- Recent Developments in the Chemistry of Natural Phenolic Compounds: Proceedings of the Plant Phenolics Group SymposiumFrom EverandRecent Developments in the Chemistry of Natural Phenolic Compounds: Proceedings of the Plant Phenolics Group SymposiumW. D. OllisNo ratings yet
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- Experiment 1: Separation and Identification of CationsDocument6 pagesExperiment 1: Separation and Identification of CationsJoseph Pelaelo100% (1)
- Group 1 Activity 5. Literature Review Search of The Approved Research TitleDocument10 pagesGroup 1 Activity 5. Literature Review Search of The Approved Research TitleMary Heart PechaycoNo ratings yet
- PVC LS100 by LGDocument1 pagePVC LS100 by LGabhaygupta1No ratings yet
- Hybridization ThesisDocument5 pagesHybridization Thesisvxjtklxff100% (2)
- CHP 1 Suggested AnswerDocument10 pagesCHP 1 Suggested AnswerRachel Wong74% (31)
- Concrete Exposures ClassesDocument4 pagesConcrete Exposures ClasseshamidkarimpourNo ratings yet
- Reflux Extraction and Cleanup Process by Column Chromatography ForDocument7 pagesReflux Extraction and Cleanup Process by Column Chromatography Fordanish.hakeem251No ratings yet
- Formaldehyde - H2CO - PubChemDocument95 pagesFormaldehyde - H2CO - PubChemRuchita PoilkarNo ratings yet
- Standard Operating Procedure No. 30 Icp-Oes Analysis: Revision LogDocument19 pagesStandard Operating Procedure No. 30 Icp-Oes Analysis: Revision LogMayur Khalatkar100% (1)
- Document Impulse VSG VMGDocument4 pagesDocument Impulse VSG VMGBiriba_nitNo ratings yet
- Experiment I: Determination of Iron (II) in Mohr Salt Solution Using Potassium DichromateDocument8 pagesExperiment I: Determination of Iron (II) in Mohr Salt Solution Using Potassium DichromateayushmanNo ratings yet
- Loose Nanofiltration-Based Electrodialysis For Highly Efficient Textile WastewaterDocument34 pagesLoose Nanofiltration-Based Electrodialysis For Highly Efficient Textile Wastewatersowmya SNo ratings yet
- Taxation of Business Entities 2017 8th Edition Spilker Solutions ManualDocument25 pagesTaxation of Business Entities 2017 8th Edition Spilker Solutions ManualLukeCamerondepo100% (34)
- Tyfo WS Epoxy Data Sheet (12-17)Document2 pagesTyfo WS Epoxy Data Sheet (12-17)Jhonel Loyola MalonzoNo ratings yet
- PosterDocument1 pagePoster07vnkls2qNo ratings yet
- Film-Screen Radiography-PhysicsDocument45 pagesFilm-Screen Radiography-PhysicsFouzia NoorNo ratings yet
- PaintsCoatings - Catalogue - 2023 FormuleDocument19 pagesPaintsCoatings - Catalogue - 2023 FormuleAchour BouchefraNo ratings yet
- Material Properties 17-4PHDocument6 pagesMaterial Properties 17-4PHr071040bNo ratings yet
- Pre-Prelims Revision PaperDocument6 pagesPre-Prelims Revision PaperaaaaNo ratings yet
- Physical Science Q1 Module 6Document25 pagesPhysical Science Q1 Module 6Zeporah OrdonNo ratings yet
- HPLC-ELSD Method for Triacylglycerol Analysis of Fats and OilsDocument7 pagesHPLC-ELSD Method for Triacylglycerol Analysis of Fats and OilsAlvin GunadiNo ratings yet
- D Block Jeemain - GuruDocument7 pagesD Block Jeemain - GuruAbdelfattah oufNo ratings yet
- Corrosion and Polarization Characteristics of ZincDocument9 pagesCorrosion and Polarization Characteristics of ZincMarco Miranda RodríguezNo ratings yet
- 4174 10399 1 PBDocument15 pages4174 10399 1 PBtoniNo ratings yet
- Metal Organic Framework (MOF) : CH-103 B.Tech. Chemistry Course Indian Institute of Technology IndoreDocument37 pagesMetal Organic Framework (MOF) : CH-103 B.Tech. Chemistry Course Indian Institute of Technology IndoreRhombiNo ratings yet
- Narasimlu PublicationDocument13 pagesNarasimlu Publicationsreedhar_vkNo ratings yet
- Soy - Based Flexographic Ink For Linerboard PrintingDocument63 pagesSoy - Based Flexographic Ink For Linerboard PrintingAhmed EldeebNo ratings yet
- Lydia 1-S2.0-S0022391320301669-MainDocument9 pagesLydia 1-S2.0-S0022391320301669-MainCherifNo ratings yet
- _EXP 9 KUT101Document17 pages_EXP 9 KUT101hannan sharizalNo ratings yet
- Declaration Acc. REACHDocument65 pagesDeclaration Acc. REACHJulioNo ratings yet