Professional Documents
Culture Documents
Act 5
Act 5
Uploaded by
Hannah KateOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Act 5
Act 5
Uploaded by
Hannah KateCopyright:
Available Formats
Name:_______________________________________ Course:____________________ Score:
Date:________________________________________Group No:__________________
ACTIVITY 5
BUFFERING ACTION OF THE BLOOD SERUM
Buffer action is the ability of the buffer solution to resist the changes in pH value on the
addition of small amount of an acid or a base is known as buffer action. Human blood contains a
buffer of carbonic acid (H2CO3) and bicarbonate anion (HCO3-) in order to maintain blood pH
between 7.35 and 7.45, as a value higher than 7.8 or lower than 6.8 can lead to death. In this
buffer, hydronium and bicarbonate anion are in equilibrium with carbonic acid. Furthermore, the
carbonic acid in the first equilibrium can decompose into CO2 gas and water, resulting in a
second equilibrium system between carbonic acid and water. Because CO2 is an important
component of the blood buffer, its regulation in the body, as well as that of O2 , is extremely
important. The effect of this can be important when the human body is subjected to strenuous
conditions.
Buffer solutions are extremely important in biology and medicine because most
biological reactions and enzymes need very specific pH ranges in order to work properly.
Objectives:
1. To observe the buffering action of blood serum
2. To determine the pH of blood serum
Materials/Reagents:
20ml distilled water 1 graduated cylinder centrifuge machine
1ml 0.1M NaOH 10 test tubes medicine dropper
1ml 0.1M HCl 1 test tube rack alcohol
1ml phenolphthalein 1 syringe 1 tourniquet
1ml methyl orange 5 cotton
Procedure
A. 1. Withdraw 5ml venous blood (make sure to follow aseptic technique) and place it into a test
tube. Centrifuge the sample.
2. Extract at least 1mL of serum from the blood and use it to procedure B.
B. 1. Prepare four test tubes as follows:
Test Tubes 1 2 3 4
Water 5mL 5mL 4.5mL 4.5mL
0.1 M NaOH 1 Drop - - -
0.1 M HCl - 1 Drop - -
phenolphthalein 1 Drop - 1 Drop -
Methyl orange - 1 Drop - 1 Drop
Serum - - 0.5mL 0.5 mL
2. To test tube 3, add 0.1M NaOH dropwise until it matches the color of test tube 1.
3. To test tube 4, add 0.1M HCl dropwise until it matches the color of test tube 2.
4. Record the total number of drops of 0.1M NaOH and 0.1M HCl in each case.
Biochemistry Laboratory| ACT 5 | 1
Name:_______________________________________ Course:____________________ Score:
Date:________________________________________Group No:__________________
ACTIVITY 5 PRE LAB OUTPUT:
Reminders: Answer & submit this on the day that is given by the instructor.
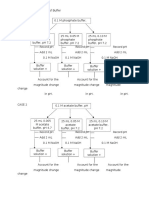
Create a Schematic Diagram from the Procedure Above:
Guide Questions:
1. What is acid-base indicator and how it is used in this activity?
Acid–base indicators are compounds that change color when they become protonated or deprotonated.
Because this color change occurs over a specific pH range, indicators can be used to approximate the
equivalence point of an acid–base titration. It is used to determine the pH of blood serum in this
activity.
2. Give at least two factors that would account for the buffering action of blood serum.
_________________________________________________________________________
_________________________________________________________________________
_________________________________________________________________________
3. Give the physiologic importance of buffers.
_________________________________________________________________________
_________________________________________________________________________
_________________________________________________________________________
4. What are the symptoms if the blood pH becomes lower or becomes higher than its
normal value?
_________________________________________________________________________
_________________________________________________________________________
_________________________________________________________________________
REFERENCES:
Biochemistry Laboratory| ACT 5 | 2
Name:_______________________________________ Course:____________________ Score:
Date:________________________________________Group No:__________________
Acid–base indicators. (n.d.). Khan Academy. Retrieved September 28, 2022, from
https://www.khanacademy.org/science/ap-chemistry-beta/x2eef969c74e0d802:acids-and-
bases/x2eef969c74e0d802:acidbase-titrations/v/acid-base-indicators#:%7E:text=Acid
%E2%80%93base%20indicators%20are%20compounds,of%20an%20acid
%E2%80%93base%20titration.
Chemistry of buffers and buffers in our blood (article). (n.d.). Khan Academy. Retrieved
September 28, 2022, from https://www.khanacademy.org/test-prep/mcat/chemical-
processes/acid-base-equilibria/a/chemistry-of-buffers-and-buffers-in-blood#:
%7E:text=Buffering%20system%20of%20blood&text=When%20any%20acidic
%20substance%20enters,of%20blood%20from%20becoming%20acidic.
https://www.med.unc.edu/pharm/sondeklab/wp- What Is Alkalosis? (2021, June 24). WebMD.
Retrieved September 28, 2022, from https://www.webmd.com/a-to-z-guides/what-is-
alkalosis
content/uploads/sites/868/2018/10/buffers_calbiochem.pdf
____________________________________________________________________________
____________________________________________________________________________
____________________________________________________________________________
ACTIVITY 5 POST LAB OUTPUT
Reminders: Answer & submit this activity after actual experiment.
(Must be printed and answer in handwritten)
Results: (Note: The answer should be based on the actual experiment)
Test Tubes Observations
1
Biochemistry Laboratory| ACT 5 | 3
Name:_______________________________________ Course:____________________ Score:
Date:________________________________________Group No:__________________
Conclusion
____________________________________________________________________________
____________________________________________________________________________
____________________________________________________________________________
____________________________________________________________________________
____________________________________________________________________________
____________________________________________________________________________
Biochemistry Laboratory| ACT 5 | 4
You might also like
- Lab ReportDocument19 pagesLab Reportapi-394241963100% (1)
- Bio Chem Labbb EditDocument37 pagesBio Chem Labbb EditAlbert Azura100% (1)
- Laboratory Manual Qc1 1Document83 pagesLaboratory Manual Qc1 1Hannah Jean LemorenasNo ratings yet
- ASTM D 1293 - 99 Standard Test Methods For PH of Water PDFDocument9 pagesASTM D 1293 - 99 Standard Test Methods For PH of Water PDFMichael Medina100% (4)
- BioChem - Experiment 8Document8 pagesBioChem - Experiment 8Allyzha AguilarNo ratings yet
- Lab Acidimetry 2012Document2 pagesLab Acidimetry 2012Adna Ivan ArdianNo ratings yet
- Practical Manual of Analytical ChemistryFrom EverandPractical Manual of Analytical ChemistryRating: 4.5 out of 5 stars4.5/5 (3)
- Amino Acid TitrationDocument9 pagesAmino Acid TitrationuğurNo ratings yet
- GECHML Expt02 Acids and BasesDocument6 pagesGECHML Expt02 Acids and BasesAngela ParaisoNo ratings yet
- Biochemistry Lab Guide OBEDocument107 pagesBiochemistry Lab Guide OBEKathlyn Patricia RealNo ratings yet
- Acid Neutralizing Capacity of An AntacidDocument4 pagesAcid Neutralizing Capacity of An AntacidibdpNo ratings yet
- Titration - Lab-ManualDocument9 pagesTitration - Lab-ManualVN BomXanhNo ratings yet
- Devices and Reagents: Burettes, Volumetric Flasks For 100 and 250 ML, TubesDocument2 pagesDevices and Reagents: Burettes, Volumetric Flasks For 100 and 250 ML, TubesAnujNo ratings yet
- Chapter 4 Lab Titration of Hydrochloric Acid With Sodium Hydroxide PDFDocument4 pagesChapter 4 Lab Titration of Hydrochloric Acid With Sodium Hydroxide PDFMara ScisciNo ratings yet
- Experiment 3: Preparing SolutionDocument4 pagesExperiment 3: Preparing SolutionÇiğdem DÜLGERBAKİNo ratings yet
- TritationDocument9 pagesTritationapi-299017686100% (1)
- Acid Base TitrationDocument5 pagesAcid Base Titrationapi-336571203No ratings yet
- PHT 414Document20 pagesPHT 414Yuppie RajNo ratings yet
- Acid Neutralizing Capacity of An Antacid: BackgroundDocument5 pagesAcid Neutralizing Capacity of An Antacid: BackgroundNabilah HarisNo ratings yet
- Lab Exp No.4Document4 pagesLab Exp No.4Jennefer TonizaNo ratings yet
- 12 - Clear Soda Titration Lab 2023Document4 pages12 - Clear Soda Titration Lab 2023Mathar BashirNo ratings yet
- Acid/Base Chemistry: Titration Lab: What Is A Titration?Document7 pagesAcid/Base Chemistry: Titration Lab: What Is A Titration?Barça LaNo ratings yet
- Laboratory Activity No 3Document6 pagesLaboratory Activity No 3Bok MatthewNo ratings yet
- THanna StudentversionDocument10 pagesTHanna StudentversionMahesh KhamitkarNo ratings yet
- Expt 1 ADocument4 pagesExpt 1 AGracelle AnneNo ratings yet
- First Pre-Lab: Spectrophometric Determination of Iron Rojanjanbaglou-101246204 Ta: Mahsabahadoori Date of Submit: 2021 - 22Th SeptemberDocument3 pagesFirst Pre-Lab: Spectrophometric Determination of Iron Rojanjanbaglou-101246204 Ta: Mahsabahadoori Date of Submit: 2021 - 22Th Septemberrozhan janbaglooNo ratings yet
- Preparation of A Naoh Standard Solution Using Direct TitrationDocument4 pagesPreparation of A Naoh Standard Solution Using Direct TitrationMuhammad jawadNo ratings yet
- Acid or Base LabDocument6 pagesAcid or Base LabMohammed MohsinNo ratings yet
- Titration Lab ReportDocument13 pagesTitration Lab Reportapi-341133750No ratings yet
- Biochem Lab Act PrelimDocument20 pagesBiochem Lab Act PrelimcharlesNo ratings yet
- Bio - PH LabDocument4 pagesBio - PH Labapi-264731832No ratings yet
- LAS 6 Preparation and Standardization of 1N Sulfuric Acid Solution 1Document3 pagesLAS 6 Preparation and Standardization of 1N Sulfuric Acid Solution 1Ann Jonneth Perino RicoNo ratings yet
- PH and BuffersDocument4 pagesPH and BuffersNirmalya Chatterjee100% (1)
- Titulacao de Acido FosforicoDocument4 pagesTitulacao de Acido FosforicoKiany SirleyNo ratings yet
- Laboratory Experiment #1common Laboratory Operations (Part 2)Document11 pagesLaboratory Experiment #1common Laboratory Operations (Part 2)Monica RilveriaNo ratings yet
- Phosphoric Acid PDFDocument4 pagesPhosphoric Acid PDFFlex GodNo ratings yet
- Titration. Lab - StudentDocument5 pagesTitration. Lab - Studentshoaib2769504No ratings yet
- Name Shatanshu Raj Student ID: SE21UCSE199 Batch Number: A4Document8 pagesName Shatanshu Raj Student ID: SE21UCSE199 Batch Number: A4Kadarla ShashankNo ratings yet
- T I T R A T I o N Expt 4Document5 pagesT I T R A T I o N Expt 4Mikhail Vander Nikolanovich VladimyrNo ratings yet
- Balancesph Meters - Acid Base Titrations With Balances LabDocument6 pagesBalancesph Meters - Acid Base Titrations With Balances LabKim Shyen BontuyanNo ratings yet
- Chem Lab Report 2Document10 pagesChem Lab Report 2api-3105312910% (1)
- Lab 2 - KHP - Volumetric AnalysisDocument5 pagesLab 2 - KHP - Volumetric AnalysisFiza MohdNo ratings yet
- Chem 131A: PH and Buffers ExperimentDocument4 pagesChem 131A: PH and Buffers ExperimentLinh NguyễnNo ratings yet
- PH, Buffer, and Dissociation ConstantDocument5 pagesPH, Buffer, and Dissociation ConstantAlisher AbdugalimovNo ratings yet
- Chem 26.1 Lab ManualExpts3-5Document18 pagesChem 26.1 Lab ManualExpts3-5Dam Yeo WoolNo ratings yet
- ACID Titr LabDocument4 pagesACID Titr LabEbuka EfobiNo ratings yet
- Acid Rain IIDocument3 pagesAcid Rain IIMaxWittNo ratings yet
- Hach 8009 Zinc Ed 08Document6 pagesHach 8009 Zinc Ed 08Beth AlvaradoNo ratings yet
- (An Exclusive Biotechnology Institute) : Training Report inDocument49 pages(An Exclusive Biotechnology Institute) : Training Report inDivya SharmaNo ratings yet
- Laboratory Plan 1:: Standardization of Sodium Hydroxide (Naoh) With Potassium Hydrogen Phthalate (KHP)Document4 pagesLaboratory Plan 1:: Standardization of Sodium Hydroxide (Naoh) With Potassium Hydrogen Phthalate (KHP)Alliza Kaye CasullaNo ratings yet
- LTS HPLC Experiment ProtocolDocument9 pagesLTS HPLC Experiment ProtocolShubhamMalikNo ratings yet
- Experiment No. 1 - Acids Bases and Buffers 1Document2 pagesExperiment No. 1 - Acids Bases and Buffers 1Raven GoseNo ratings yet
- HPLC of Caffeine and TheobromineDocument11 pagesHPLC of Caffeine and TheobromineshannonkhaneyNo ratings yet
- Biochemistry LaboratoryDocument13 pagesBiochemistry LaboratoryburgosmjvNo ratings yet
- Doc316 53 01113Document6 pagesDoc316 53 01113pothanNo ratings yet
- PhosphateDocument8 pagesPhosphateUmi NazaliaNo ratings yet
- Honors Acid Base Titration Lab 2Document4 pagesHonors Acid Base Titration Lab 2Juan Jose Granados GutierrezNo ratings yet
- Analysis of Aspirin Tablet PDFDocument5 pagesAnalysis of Aspirin Tablet PDFAnonymous TmwS3ZmseNo ratings yet
- Taller Trad de 8 Determinaciones de Parametros de Calidad de AguaDocument11 pagesTaller Trad de 8 Determinaciones de Parametros de Calidad de AguaChristian RiveraNo ratings yet
- Ultra-High Performance Liquid Chromatography and Its ApplicationsFrom EverandUltra-High Performance Liquid Chromatography and Its ApplicationsNo ratings yet
- Xi 7 GTP TELDocument24 pagesXi 7 GTP TELdanielyskim1119No ratings yet
- Buffer-Titration-Equilibrium Practice ProblemsDocument18 pagesBuffer-Titration-Equilibrium Practice ProblemssbelodoNo ratings yet
- Ionic Equilibria Questions Set 2 2 PDFDocument4 pagesIonic Equilibria Questions Set 2 2 PDFdanielmahsaNo ratings yet
- Ionic Equilibrium IDocument12 pagesIonic Equilibrium IGadde Gopala KrishnaNo ratings yet
- Chapter 21 NeutralizationDocument24 pagesChapter 21 Neutralizationronayme29No ratings yet
- Katalog Bahan Kimia 2018Document15 pagesKatalog Bahan Kimia 2018Kristo RickyNo ratings yet
- Lab Manual - Practical 5 - Determination of Buffer CapacityDocument3 pagesLab Manual - Practical 5 - Determination of Buffer Capacitysandi fernando100% (1)
- EnzEng 2 EnzymeKinetics C V VIIDocument43 pagesEnzEng 2 EnzymeKinetics C V VIIEkuino Simanungkalit100% (1)
- Standard Methods For The Examination of Water and WastewaterDocument8 pagesStandard Methods For The Examination of Water and WastewaterMithy GilNo ratings yet
- GFS Chemicals CartDocument1 pageGFS Chemicals CartOmar SaaedNo ratings yet
- BufferDocument25 pagesBuffernaghma KhanNo ratings yet
- 5: PH Measurement and Its Applications (Experiment) : ObjectivesDocument19 pages5: PH Measurement and Its Applications (Experiment) : ObjectivesNajmi NasirNo ratings yet
- Heparins, Low-Molecular-MassDocument3 pagesHeparins, Low-Molecular-MassArtem KulikovNo ratings yet
- P53 HachDocument99 pagesP53 HachGANESWARAN RaathaNo ratings yet
- Practical Manual in Biochemistry and Clinical BiochemistryDocument6 pagesPractical Manual in Biochemistry and Clinical BiochemistryYan Petrov100% (1)
- Physiology of Acid Base Balance by Dr. ROOMIDocument70 pagesPhysiology of Acid Base Balance by Dr. ROOMIMudassar Roomi100% (1)
- SchematicDocument4 pagesSchematicCai PascualNo ratings yet
- General Chemistry 2 Final Exam ReviewerDocument6 pagesGeneral Chemistry 2 Final Exam ReviewerZyriel SaavedraNo ratings yet
- Investigating The Factors That Affect Enzyme Activity and Their Corresponding EffectsDocument6 pagesInvestigating The Factors That Affect Enzyme Activity and Their Corresponding EffectsAIra OrtegaNo ratings yet
- Air Receivers Volume CalculationDocument83 pagesAir Receivers Volume CalculationsudarwantoNo ratings yet
- Full TextDocument6 pagesFull TextSachin PatilNo ratings yet
- Practice Problems For KSP - ChemistryDocument12 pagesPractice Problems For KSP - Chemistrybutterflybre0% (1)
- 14 Acid Base Equilibria Iedxcel PDFDocument9 pages14 Acid Base Equilibria Iedxcel PDFHappy AyichNo ratings yet
- PowerEdge Sizer UserGuide v2Document27 pagesPowerEdge Sizer UserGuide v2Trương ĐứcNo ratings yet
- Reagent ManualDocument23 pagesReagent ManualAli RazaNo ratings yet
- Equilibrium Notes and Solved ExerciseDocument9 pagesEquilibrium Notes and Solved ExerciseSANKAR VNo ratings yet
- 17bufferkspap 100308200536 Phpapp01Document235 pages17bufferkspap 100308200536 Phpapp01Isabelle AbadNo ratings yet
- ABGsDocument9 pagesABGsafejjhNo ratings yet