Professional Documents
Culture Documents
Science Component 3
Science Component 3
Uploaded by
shilpi pradhanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Science Component 3
Science Component 3
Uploaded by
shilpi pradhanCopyright:
Available Formats
SCIENCE COMPONENT 3 (WRITE UP)
How is ‘altitude’ different to sea level?
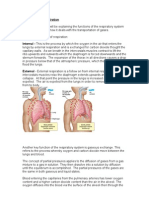
Air is comprised of different molecules, with nitrogen (79.04%) and oxygen (20.93%) making up the majority of each breath we take.
This composition of air remains consistent, whether we are at sea level or at altitude.
However, with altitude, the “partial pressure” of oxygen in this air (how many molecules of oxygen are in a given volume of air) changes.
At sea-level, the partial pressure of oxygen is 159 mmHg, whereas at 8,848m above sea level (the summit of Mt Everest), the partial
pressure of oxygen is only 53 mmHg.
At high altitudes, oxygen molecules are further apart because there is less pressure to “push” them together. This effectively means
there are fewer oxygen molecules in the same volume of air as we inhale. In scientific studies, this is often referred to as “hypoxia”.
What happens in the body in high altitudes?
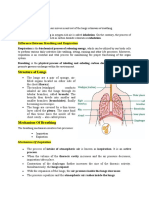
Within seconds of exposure to altitude, ventilation is increased, meaning we start trying to breathe more, as the body responds to less
oxygen in each breath, and attempts to increase oxygen uptake. Despite this response, there’s still less oxygen throughout your
circulatory system, meaning less oxygen reaches your muscles. This will obviously limit exercise performance.
Within the first few hours of altitude exposure, water loss also increases, which can result in dehydration. Altitude can also increase your
metabolism while suppressing your appetite, meaning you’ll have to eat more than you feel like to maintain a neutral energy balance.
When people are exposed to altitude for several days or weeks, their bodies begin to adjust (called “acclimation”) to the low-oxygen
environment. The increase in breathing that was initiated in the first few seconds of altitude exposure remains, and haemoglobin levels
(the protein in our blood that carries oxygen) increase, along with the ratio of blood vessels to muscle mass.
You might also like
- M Eteor Ology An D Ocean Ogr Ap Hy: Atm Ospheric PressureDocument13 pagesM Eteor Ology An D Ocean Ogr Ap Hy: Atm Ospheric PressureRegine A. AnsongNo ratings yet
- Process of BreathingDocument3 pagesProcess of BreathingssmaddiNo ratings yet
- CHAPTER 14 Cabin Atmosphere ControlDocument47 pagesCHAPTER 14 Cabin Atmosphere Controlখালিদহাসান60% (5)
- BreathingDocument5 pagesBreathingGelyn Lanuza-AsuncionNo ratings yet
- Breathing: Breathing Is The Process That Moves Air in and Out of The LungsDocument5 pagesBreathing: Breathing Is The Process That Moves Air in and Out of The LungsArief Rachman WiladisastraNo ratings yet
- Lab Report Bio Exp 1Document3 pagesLab Report Bio Exp 1NurulHaidah100% (4)
- 4.2 - Respiratory SystemDocument10 pages4.2 - Respiratory SystemBenJenkinsNo ratings yet
- Function of The Respiratory SystemDocument5 pagesFunction of The Respiratory SystemRachelHatcher123No ratings yet
- Acclimatization To AltitudeDocument6 pagesAcclimatization To AltitudeEugene Lucino CodisNo ratings yet
- 11 Lung PhysiologyDocument14 pages11 Lung PhysiologyGlen UrbaniNo ratings yet
- Respiratory SystemDocument7 pagesRespiratory Systemrienz nicnic peraltaNo ratings yet
- Exercise in Hypobaric, Hyperbaric and Microgravity EnvironmentsDocument35 pagesExercise in Hypobaric, Hyperbaric and Microgravity EnvironmentsdeepuphysioNo ratings yet
- Altitude Physiology: PRESENTED BY Dick WilliamsDocument52 pagesAltitude Physiology: PRESENTED BY Dick WilliamsDeepak ArkalgudNo ratings yet
- Atmospheric PressureDocument14 pagesAtmospheric PressureIsabel FilanNo ratings yet
- Evens RespiortyDocument4 pagesEvens RespiortyamyhowarthNo ratings yet
- Recall The Functions of The Organs in The Gas Exchange SystemDocument14 pagesRecall The Functions of The Organs in The Gas Exchange SystemAhmad AhmadNo ratings yet
- RespirationDocument7 pagesRespirationMemona KhalidNo ratings yet
- Gaseous Exchange in HumanDocument14 pagesGaseous Exchange in HumanIzzul Syahmi100% (1)
- Document 5Document5 pagesDocument 5kauthar hassanNo ratings yet
- Respiration in OrganismDocument67 pagesRespiration in Organismsangeetha alurNo ratings yet
- Altitude PhysiologyDocument41 pagesAltitude Physiologyshepherd jeremiahNo ratings yet
- Gas Exchange in HumansDocument5 pagesGas Exchange in HumansRAGHU RAM SAKAMURINo ratings yet
- Chemistry Signature Assignment and ReflectionDocument4 pagesChemistry Signature Assignment and Reflectionapi-291557491No ratings yet
- Cells of Animals: Glucose + Oxygen Carbon Dioxide + Water + EnergyDocument3 pagesCells of Animals: Glucose + Oxygen Carbon Dioxide + Water + EnergyHONLETH ROMBLONNo ratings yet
- Respiratory SystemDocument25 pagesRespiratory SystemAnonymous 5hYhEkINo ratings yet
- Chapter 11 BioDocument18 pagesChapter 11 BioRylee LNo ratings yet
- Post Lab Question Experiment 6, 7, 8 (Farah)Document3 pagesPost Lab Question Experiment 6, 7, 8 (Farah)SkefadiutoNo ratings yet
- Assignment Respiratory During ExerciseDocument3 pagesAssignment Respiratory During Exercisedomhughes1093No ratings yet
- Exercise in Difference EnvironmentDocument7 pagesExercise in Difference EnvironmentMozil Fadzil KamarudinNo ratings yet
- Diving Fizzyology R. Pyle IANTDDocument15 pagesDiving Fizzyology R. Pyle IANTDcaptain essamNo ratings yet
- respiration experimentDocument4 pagesrespiration experimentTanieka PowellNo ratings yet
- Human PerformanceDocument8 pagesHuman PerformanceRamNo ratings yet
- Physiology of Respiration 2Document31 pagesPhysiology of Respiration 2kuhutansittinurhalizaNo ratings yet
- Anatomi Fisiologi PernapasanDocument87 pagesAnatomi Fisiologi PernapasanAndre ChundawanNo ratings yet
- Respiratory SystemDocument6 pagesRespiratory SystemHamza MinongNo ratings yet
- Mechanics of BreathingDocument5 pagesMechanics of BreathingMark Lyndon M OrogoNo ratings yet
- Post Lab Question Experiment 6, 7, 8 (Farah)Document3 pagesPost Lab Question Experiment 6, 7, 8 (Farah)Skefadiuto100% (2)
- 6.4 QuestionsDocument18 pages6.4 QuestionsAurelija GardžiulytėNo ratings yet
- Biology - Respiration & The Respiratory SystemDocument2 pagesBiology - Respiration & The Respiratory SystemMegan TaylorNo ratings yet
- Breathing During Sleep-TPO24-R2Document4 pagesBreathing During Sleep-TPO24-R2hhpqjebhooNo ratings yet
- What Is Atmospheric Pressure?Document1 pageWhat Is Atmospheric Pressure?Aditya SarmaNo ratings yet
- Respiratory Powerpoint GaDocument28 pagesRespiratory Powerpoint Gakevinramirezzz365No ratings yet
- Respiratory System Structure and FunctionDocument6 pagesRespiratory System Structure and FunctionRavi BhollahNo ratings yet
- Biology ScienceDocument9 pagesBiology SciencetalaNo ratings yet
- The Respiratory SystemDocument2 pagesThe Respiratory SystemRaluca MoldovanNo ratings yet
- Reflection Respiratory SystemDocument4 pagesReflection Respiratory SystemAin Sufiza0% (1)
- Physiology of RespirationDocument2 pagesPhysiology of RespirationIOSRjournalNo ratings yet
- Breathing LabDocument3 pagesBreathing Labiheartfood100% (2)
- Autonomic Breathing How Ventilation Is RegulatedDocument2 pagesAutonomic Breathing How Ventilation Is Regulatedrienz nicnic peraltaNo ratings yet
- File 1667629685 GUSBAS202232069 UpdatedRespiration-module2Document4 pagesFile 1667629685 GUSBAS202232069 UpdatedRespiration-module2pranjal dubeyNo ratings yet
- Respiratory SystemDocument7 pagesRespiratory SystemLinh LeNo ratings yet
- As PE Lesson 27 Resp Syst 2013-14Document13 pagesAs PE Lesson 27 Resp Syst 2013-14mancollNo ratings yet
- What Is Respiration?Document8 pagesWhat Is Respiration?Waleed Bin KhalidNo ratings yet
- ch17Document7 pagesch17Akanksha AvasthiNo ratings yet
- Normal Oxygen Transport: Susanne A ClarkDocument256 pagesNormal Oxygen Transport: Susanne A Clarkghoncheh0% (1)
- Resdpiratory System NotesDocument1 pageResdpiratory System NotesnanaNo ratings yet
- Gas Exchange NewDocument30 pagesGas Exchange Newmariaamkhaann22No ratings yet
- Chapter 11 Bio NotesDocument4 pagesChapter 11 Bio NotesArabella ShepherdNo ratings yet
- Olugbade Mercy Physiology AssignmentDocument4 pagesOlugbade Mercy Physiology Assignmentolugbaded3No ratings yet