Professional Documents
Culture Documents
BMM LEC 5 SN Structure & Function of Amino Acids, Peptides & Proteins
Uploaded by
SARAH SAFIAH TAJUL ARIFFINOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
BMM LEC 5 SN Structure & Function of Amino Acids, Peptides & Proteins
Uploaded by
SARAH SAFIAH TAJUL ARIFFINCopyright:
Available Formats
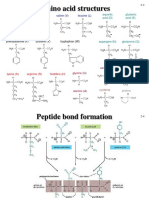
Structure of Amino Acid
Amino Acid is the building block of proteins B. Polar, uncharged side chain
-Two functional groups: amino 1.Hydrophilic : Zero charged at neutral pH
(NH2) and carboxyl groups 2.Contain:
(COOH). i.Polar OH group
-R group (side chain) determines -Participate in hydrogen bond with water
amino acid its unique structure -site of attachment for structure , ie phosphate group
and properties. ii.sulfhydryl group (SH) - Disulfide bond - active sites of enzymes
a. Stereoisomers 3. Location: interior of membrane associated proteins
carbon chiral : Exist as enantiomers (mirror image of one another) – L and D C. Acidic side chain- negative charged
forms. Only L isomers used as building block in protein 1.Proton donors
b. Amphoteric Molecule – behaving as both acid & base 2.Contain carboxylate group (COO-)
3.Location: interior of membrane associated proteins
D. Basic side chain- positive charged
1.Accept protons (protonated)
2.Location: interior of membrane associated proteins
Why you should know the different type of aa?
-contribution to the formation various bond ie
hydrophobic, electrostatic and Van der Waal‟s
interactions to establish protein conformation.
Human genetic code directly encodes 20 a.a.
c. Exist as Zwitterions All have a common structure except for the R group
d. Has it’s own pK values
•pH at which amino group or carboxyl group 50% ionises.
•pk1 of carboxylic group of all aa is ~ 2 (1.8-2.4) - acidic
•Pk2 of all amino groups is ~9.5 (8.8-11.0) – base PEPTIDES
e. Has it’s own isoelectric point Amino acids are joined by the peptide bond to form peptide chains.
Isoelectric Point (pI): pH at which an Peptide, dipeptide, tripeptide (Gluthathione), oligopeptide and polypeptide
amino acid has no NET charge The main chain of polypeptide is sometimes termed backbone
Classification of amino acid A) Polypeptide chain
•Based on the properties of side chain:
-A sequence of amino acid.
1)Non polar side chain
-Polypeptide chains have direction:
2)Polar, uncharged side chain
3)Acidic side chain Polar, Charged •Begin: Amino terminal
4)Basic side chain •End: carboxyl terminal
- Polarity – unequal/uneven distribution -Example: Insulin (86 amino acids)
charges on the two ends of any PROTEIN STRUCTURE
molecule(ie H20), hydrophilic
1. Primary Structure : Unique
A. Non polar side chain
1.Does not bind or donate
sequence of amino acid
protons/participate in hydrogen or Sequence determined during
ionic bonds. DNA replication
2.So cannot form hydrogen bond with A slight change in primary
water. structure can affect a
3.Promotes hydrophobic interactions – protein’s conformation and
stabilizing protein structure
ability to function
4.Location: on surface of membrane
2. Secondary Structure :
proteins, interacts with lipid
Polypeptide chain can fold into
–Coils = alpha helix
–Lie parallel to each other
=beta pleated sheet
•Hydrogen bonds at regular
intervals along the polypeptide
backbone
a) Secondary structure: The α-helix COLLAGEN
Hydrogen bonds form between NH (amide hydrogen) and CO Most abundant protein in human
(carbonyl oxygen) group in the same strand Three polypeptides : alpha-helix
The α-helix coils at every 4th amino acid Consist glycine, hydroxylproline (x),
b) Secondary Structure: β- Pleated Protein hydroxylysine(y)
H+ bond is formed b/w amino and co gp on an adjacent strand. Aggregation of collagen into long fiber:
Polypeptide strand lie in the same direction: parallel tough and tensile strength
Polypeptide strand lie in the opposite direction: antiparallel b. Globular Protein The importance of primary structure
3. Tertiary Structure : Refers to the helical form coiled into a ball •Form functional components of the cells Determined by the nucleotide sequence in
like structure = 3D structure of protein •They are compactly folded and coiled. the gene for the polypeptide or protein.
The functional unit called the domain. •Examples are: Determines the 3-D conformation through
The folding of a protein into its domains is related to the -insulin,plasma albumin,globulin, enzymes folding patterns
hydrophilic or hydrophobic properties of its amino acids. 2. Classification based on composition: A slight change in primary structure can
Example : myoglobin = Single polypeptide chain of 154 aa a. Simple protein: Composed of entirely aa. affect tertiary conformation of the cells
The forces that hold 3º structure together are: -Example: and ability to function
a.van der Waals interaction d.ionic i. Albumin, Globulin Example: Sickle Cell Anemia
b.Hydrogen-bonds e.disulphide linkages - present in egg, milk and blood Abnormal hemoglobin formation because
c.hydrophobic interactions -High biological value i.e. contain all of a single amino acid substitution.
MYOGLOBIN essential amino acids and easily digested. causes hemoglobin to crystallize,
Single polypeptide unit that form into several alpha helixes (folded) = 3D ii. Globulins (Histones): deforming the red blood cells and leading
structure, |154 amino acid |Attach to heme -Basic proteins rich in histidine amino acid to clogs in blood vessels.
Function: Oxygen storage in muscle -Combine with DNA
4. Quaternary Structure : Refers to more than one ball like globular α1 globulin: antitrypsin: protease inhibitor
structures held together by weak interactions α2 globulin: haptoglobin: protein that
Two or more polypeptide chains join in aggregate, they form a binds hemoglobin to prevent its excretion
quaternary structure; eg hemoglobin, DNA polymerase. by the kidney
Often quaternary proteins are complexed with a different β-globulin: transferrin: protein that
molecule; ie a mineral. Hemoglobin contains iron, for example. transport iron
Hemoglobin structure : 2 alpha and 2 γ-globulins = Immunoglobulins
beta subunits (antibodies): responsible for immunity
Alpha unit= 146 amino acid, beta b. Complex or Conjugated Proteins
unit = 141 amino acid Made up of aa & other organic compound
each chain is associated w 1 heme gp The non-aa group is termed as the
Function: Oxygen transportation prosthetic group.
c. Derived Protein
denatured proteins that are derived from
simple & conjugated proteins that occur
after inactive proteins & enzymes are
broken down initially to peptides by
intracellular proteolytic enzymes.
then degraded to amino acids within the
Classification of proteins: a.Fibrous Protein cell for re-synthesis of new proteins.
1.Based on shape (structural): form structural comp in the body
a.Fibrous protein Exhibit special mechanical
b.Globular protein properties = unique structure but
2.Based on composition : simple
a.Simple Long, rod-shape molecules,
b.Conjugate
Spiral and helical and are cross
c.Derived protein
linked by disulfide & hydrogen bond
3.Based on biological function
Physically tough
a. Movement
•Example:
b. Structure
1.Collagen: skin, cartilage, ligament
c. Signaling
2.Keratin: hair, skin, nails
d. Transport
3.Elastin: Wall or blood vessels
e. Catalysis
4.Fibrin: blood clots
f. Protection
You might also like
- BIO 329 Cellular and Molecular Biology Proteins - Structure and FunctionsDocument39 pagesBIO 329 Cellular and Molecular Biology Proteins - Structure and FunctionsCaleb HeNo ratings yet
- MCAT - BiologyDocument15 pagesMCAT - BiologyEmily Teo100% (1)
- 1.4 Proteins-1Document54 pages1.4 Proteins-1Ng Kei CheongNo ratings yet
- B. β pleated sheet = a parallel or antiparallel: ProteinsDocument17 pagesB. β pleated sheet = a parallel or antiparallel: ProteinsJackielou MaquisoNo ratings yet
- Introduction To Biochemistry Instructor: Bita ZamiriDocument17 pagesIntroduction To Biochemistry Instructor: Bita ZamiriMatthew GhaffariNo ratings yet
- Characteristics of Carbon: PhosphateDocument7 pagesCharacteristics of Carbon: Phosphate둡챙브로No ratings yet
- Rhettbro Amino Acid Protein StructureDocument1 pageRhettbro Amino Acid Protein StructureFatiya ShariffNo ratings yet
- ProteinDocument3 pagesProteinJovan SernaNo ratings yet
- 2022.09.07 Protein Structure and Function PT 1 (Ch4)Document24 pages2022.09.07 Protein Structure and Function PT 1 (Ch4)Allison KwanNo ratings yet
- PROTIENSDocument2 pagesPROTIENSQsh SinoroNo ratings yet
- B 1.2 HL ProteinsDocument21 pagesB 1.2 HL ProteinsFAITH WAITHERANo ratings yet
- Biochem M1u1Document7 pagesBiochem M1u1Yvonne Nerio BadorNo ratings yet
- Primary Structure: Beta SheetDocument1 pagePrimary Structure: Beta Sheetthat/niggaNo ratings yet
- 05 - Amino Acid, Protein and Protein MetabolismDocument63 pages05 - Amino Acid, Protein and Protein MetabolismAzzarina AzreenNo ratings yet
- Chapter 3 Proteins & Proteins Purification TechniquesDocument10 pagesChapter 3 Proteins & Proteins Purification Techniquesdaniel AfiqNo ratings yet
- Protein and Nucleic AcidDocument17 pagesProtein and Nucleic AcidlinhNo ratings yet
- Amino AcidsDocument10 pagesAmino AcidsHaneen HafizNo ratings yet
- Biomedical MaterialsDocument32 pagesBiomedical MaterialsMuhammad Mushtaq AliNo ratings yet
- Lec. 4Document6 pagesLec. 4Dr. Mohamed ShamsNo ratings yet
- FOOD20003 NotesDocument42 pagesFOOD20003 NotescallensciberNo ratings yet
- Krislyn Diane Paradero Maano - BIO 024 - SESSION 7Document8 pagesKrislyn Diane Paradero Maano - BIO 024 - SESSION 7Krislyn MaanoNo ratings yet
- ProteinDocument2 pagesProteinharith r donovanNo ratings yet
- 1.6 ProteinsDocument1 page1.6 ProteinsHroaldrNo ratings yet
- ProteinsDocument12 pagesProteinsSaloni PatelNo ratings yet
- Proteins: AP BiologyDocument27 pagesProteins: AP BiologycoryguntherNo ratings yet
- Biochemistry Week 7 Structures and Functions of Proteins I-2020Document43 pagesBiochemistry Week 7 Structures and Functions of Proteins I-2020Karen 3No ratings yet
- Proteins P2Document3 pagesProteins P2Biang AbdullahNo ratings yet
- BIOCHEMISTRY LEC Module 1Document8 pagesBIOCHEMISTRY LEC Module 156bmkkn2rnNo ratings yet
- ProteinsDocument38 pagesProteinsManan PatelNo ratings yet
- Ch3 Proteins (KF)Document25 pagesCh3 Proteins (KF)julie rainesNo ratings yet
- 6 Proteins - IntroDocument25 pages6 Proteins - Introemanuel coatesNo ratings yet
- Unit 2 - 3Document29 pagesUnit 2 - 3Sunita SharmaNo ratings yet
- Cellular Organization NotesDocument6 pagesCellular Organization NotesGrayson JamesNo ratings yet
- 6 PP ProteinsDocument29 pages6 PP Proteinsqwerty123No ratings yet
- Macromolecules of The Cells PDFDocument30 pagesMacromolecules of The Cells PDFAgzar RidhoNo ratings yet
- 005 Ch03 Proteins v2020 PDFDocument4 pages005 Ch03 Proteins v2020 PDFshahidabubaker19No ratings yet
- Kaplan Biochem-QuizletDocument1 pageKaplan Biochem-QuizletAnjali PradhanNo ratings yet
- Peptide Vor Le SungDocument50 pagesPeptide Vor Le SungtigersinhaNo ratings yet
- Protein Biochemistry: Lecturer: Dr. A. BurgerDocument21 pagesProtein Biochemistry: Lecturer: Dr. A. BurgerkhekhyNo ratings yet
- Biotek 3 ProteinDocument25 pagesBiotek 3 ProteinArfan NurdinNo ratings yet
- 03 - Intro UNIT 2Document24 pages03 - Intro UNIT 2Elena OlmedoNo ratings yet
- Acid Amino NotesDocument4 pagesAcid Amino NotesCikgu RameshNo ratings yet
- Module - 1 NotesDocument50 pagesModule - 1 Notesums.fsc.2020No ratings yet
- Protein and Amino Acid Chemistry By: Dr. Hena Alcantara: BiochemistryDocument7 pagesProtein and Amino Acid Chemistry By: Dr. Hena Alcantara: BiochemistryRay Emmanuel Enriquez DomingoNo ratings yet
- ABS 311 Cell Biology: The Macromolecules of The CellDocument30 pagesABS 311 Cell Biology: The Macromolecules of The CellKelsey WhitmoreNo ratings yet
- Lec 6Document27 pagesLec 6Sreemanti DeyNo ratings yet
- Biochem MidtermsDocument6 pagesBiochem MidtermskizzaymenteraNo ratings yet
- Exam 2 Study GuideDocument28 pagesExam 2 Study GuideelizaNo ratings yet
- Topic 2: Genes and Health: Polar Hydrophilic Non-Polar HydrophobicDocument8 pagesTopic 2: Genes and Health: Polar Hydrophilic Non-Polar HydrophobicHaniNo ratings yet
- Amino Acids and Proteins ReviewerDocument5 pagesAmino Acids and Proteins ReviewerDaine MarconNo ratings yet
- Bioinorganic Chemistry LectureDocument28 pagesBioinorganic Chemistry LectureKamal ChoudhuryNo ratings yet
- Amino Acids and ProteinsDocument5 pagesAmino Acids and ProteinsGrace FernandoNo ratings yet
- Basic Inorganic Chemistry Part 3 Bioinorganic ChemistryDocument44 pagesBasic Inorganic Chemistry Part 3 Bioinorganic ChemistryKevin B. EspinocillaNo ratings yet
- Introduction To BiochemistryDocument8 pagesIntroduction To BiochemistryGELOI A.N.SNo ratings yet
- Engineering Biology: Proteins: Class II Richa SharmaDocument23 pagesEngineering Biology: Proteins: Class II Richa SharmaSwetank SahaiNo ratings yet
- Amino Acid Structures: Valine (V) Leucine (L) Methionine (M) Isoleucine (I)Document10 pagesAmino Acid Structures: Valine (V) Leucine (L) Methionine (M) Isoleucine (I)Lalu Muhammad Nadil A.No ratings yet
- Protein 3Document32 pagesProtein 3Miran El-MaghrabiNo ratings yet
- Chapter 2 Part 2Document8 pagesChapter 2 Part 2DANo ratings yet
- Basic Inorganic Chemistry Part 3 Bioinorganic ChemistryDocument44 pagesBasic Inorganic Chemistry Part 3 Bioinorganic ChemistryGuru P MNo ratings yet
- PT GM1 2014 Medic UitmDocument8 pagesPT GM1 2014 Medic UitmSARAH SAFIAH TAJUL ARIFFINNo ratings yet
- PT GM1 2013 Medic UitmDocument9 pagesPT GM1 2013 Medic UitmSARAH SAFIAH TAJUL ARIFFINNo ratings yet
- PT GM1 2015Document9 pagesPT GM1 2015SARAH SAFIAH TAJUL ARIFFINNo ratings yet
- PT GM1 2010 Medic UitmDocument7 pagesPT GM1 2010 Medic UitmSARAH SAFIAH TAJUL ARIFFINNo ratings yet
- BMM LEC 10 SN Enzymes in GlycolysisDocument5 pagesBMM LEC 10 SN Enzymes in GlycolysisSARAH SAFIAH TAJUL ARIFFINNo ratings yet
- BMM LEC 14 Oxidation of Fatty Acids SHS 2018Document36 pagesBMM LEC 14 Oxidation of Fatty Acids SHS 2018SARAH SAFIAH TAJUL ARIFFINNo ratings yet
- BMM LEC 4 SN Structure Function of Carbohydrates & LipidsDocument3 pagesBMM LEC 4 SN Structure Function of Carbohydrates & LipidsSARAH SAFIAH TAJUL ARIFFINNo ratings yet
- Biochemistry PRC 2 Data Analysis EnzymeDocument4 pagesBiochemistry PRC 2 Data Analysis EnzymeSARAH SAFIAH TAJUL ARIFFINNo ratings yet
- BMM PRACTICAL 1 Report PRC1 BMM Group Q (Senior Lepas)Document8 pagesBMM PRACTICAL 1 Report PRC1 BMM Group Q (Senior Lepas)SARAH SAFIAH TAJUL ARIFFINNo ratings yet
- Focus Life Sciences Grade 10 Learner's Boo - UnknownDocument308 pagesFocus Life Sciences Grade 10 Learner's Boo - UnknownAaliyah Ballim79% (24)
- IB Biology Notes - 32 Carbohydrates, Lipids and ProteinsDocument2 pagesIB Biology Notes - 32 Carbohydrates, Lipids and ProteinsJohn Philip D. NapalNo ratings yet
- Home Remedies For Hair Growth - Safe Natural TreatmentDocument34 pagesHome Remedies For Hair Growth - Safe Natural Treatmentkavya.noriNo ratings yet
- Midterm Exam: Faculty of Pharmacy 21 September University First YearDocument1 pageMidterm Exam: Faculty of Pharmacy 21 September University First YearstarvationNo ratings yet
- Cvb-Feed-Table-2019 (NRC Holandes) PDFDocument696 pagesCvb-Feed-Table-2019 (NRC Holandes) PDFFrederico VelascoNo ratings yet
- Secreted Giardia Intestinalis Cysteine Proteases Disrupt Intestinal Epithelial Cell JunctionalDocument17 pagesSecreted Giardia Intestinalis Cysteine Proteases Disrupt Intestinal Epithelial Cell JunctionalAura-Nicoleta PopinciucNo ratings yet
- Bio3110 - Biochemistry Ii-Intermediary Metabolism Enzyme and Enzyme KineticsDocument11 pagesBio3110 - Biochemistry Ii-Intermediary Metabolism Enzyme and Enzyme KineticsNaiomiNo ratings yet
- (Africa Human Genome Initiative Series 3) Wilmot Godfrey James, Human Sciences Research Council-Africa in The Age of Biology-HSRC Publishers (2004)Document24 pages(Africa Human Genome Initiative Series 3) Wilmot Godfrey James, Human Sciences Research Council-Africa in The Age of Biology-HSRC Publishers (2004)pradiprawat55No ratings yet
- Common Mistakes-BiologyDocument8 pagesCommon Mistakes-BiologyteahockNo ratings yet
- Lesson 2 Uses of GlucoseDocument12 pagesLesson 2 Uses of Glucosekaniewska262No ratings yet
- Nephelometry LODocument8 pagesNephelometry LOSantiagoAFNo ratings yet
- PHD Thesis MMM FinalDocument205 pagesPHD Thesis MMM FinalDeniz BaldoğanNo ratings yet
- CH 06Document6 pagesCH 06Enjie Elrassi100% (1)
- Isolation and Hydrolysis of MyoglobinDocument3 pagesIsolation and Hydrolysis of MyoglobinSean HermanNo ratings yet
- Ranjeet Rai, Manwar BansariDocument58 pagesRanjeet Rai, Manwar BansariRanjeet Rai100% (1)
- 01 Discovery of DNA and RNA - 12-10-21Document6 pages01 Discovery of DNA and RNA - 12-10-21a192062No ratings yet
- Qualitative Colour Reaction of Intact and Hydrolyzed Protein ProductsDocument4 pagesQualitative Colour Reaction of Intact and Hydrolyzed Protein ProductsKurapika Freccs ZoldyickNo ratings yet
- Scientific Posts: Csir-National Botanical Research InstituteDocument10 pagesScientific Posts: Csir-National Botanical Research InstituteYogesh KapilNo ratings yet
- Botany Ug SyllabusDocument205 pagesBotany Ug SyllabusJinu MadhavanNo ratings yet
- Test Medicina Lingua Inglese 2014 - Domande e RisposteDocument40 pagesTest Medicina Lingua Inglese 2014 - Domande e RisposteSkuola.netNo ratings yet
- UNIT-IV Lecture Notes BP205T Computer Applications in Pharmacy-Updated 07.08.2020Document31 pagesUNIT-IV Lecture Notes BP205T Computer Applications in Pharmacy-Updated 07.08.2020B.MAHAALAKSHMINo ratings yet
- Functional Properties of ProteinsDocument19 pagesFunctional Properties of ProteinsarjunikaNo ratings yet
- Biochemistry Questions and AnswersDocument28 pagesBiochemistry Questions and AnswersAgaba Moris Bogoya67% (3)
- WWW Chem Latech EduDocument6 pagesWWW Chem Latech EdumanishNo ratings yet
- A Description of The Potential Growth and Body Composition of Two Commercial Broiler StrainsDocument29 pagesA Description of The Potential Growth and Body Composition of Two Commercial Broiler StrainsFabianeNo ratings yet
- TD Bio 211 2023-2024Document9 pagesTD Bio 211 2023-2024Remadji vieriNo ratings yet
- Amino Acid: Met - Asp - Pro - Tyr - Val - THR - UAADocument5 pagesAmino Acid: Met - Asp - Pro - Tyr - Val - THR - UAAkimNo ratings yet
- Pamela Grace Tañalas - Assessment 7 PATHFIT 1Document8 pagesPamela Grace Tañalas - Assessment 7 PATHFIT 1Pamela TanalasNo ratings yet
- Premium ProductsDocument14 pagesPremium ProductsPreet SinghNo ratings yet
- Mcqs ch03Document5 pagesMcqs ch03tess_15No ratings yet