Professional Documents
Culture Documents
Khalida Mudassar 4325 (HBsAG
Uploaded by
Khalida MuddasserCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Khalida Mudassar 4325 (HBsAG
Uploaded by
Khalida MuddasserCopyright:
Available Formats
Patient Detail : Registration Location: Reference: Patient Number:
Khalida Mudassar 4325 Kot Addu CL Parco Special Project (Credit) 901301-22-11246860
Age/Sex Mobile # Registration Date: Consultant: Case Number:
51 (Y) / F - 03006370574 07-Dec-2022 14:44 PARCO ANNUAL MEDICAL 901334-07-12
Department of Chemical Pathology Reporting Time: 08-Dec-2022 02:22
Serum HBsAg
Non-Reactive
Cutoff
Value 1.00
Patient
Value 0.27
Non Reactive <1.0 | Reactive ≥ 1.0
Electronically verified report. No signature required. Lab reports should be interpreted by a physician in correlation with clinical and radiologic findings.
Interpretation:
1)It can be detected in high levels in serum during acute or chronic hepatitis B virus (HBV) infection.
2)A reactive HBsAg test indicates that the person is infectious.
3)A non-reactive HBsAg test indicates that the person is not infectious.
4)In patients who subsequently recover, HBsAg usually becomes undetectable after four to six months.
5)Persistence of HBsAg for more than six months implies chronic infection.
6)HBsAg is the antigen used to make hepatitis B vaccine. So, it can also be reactive for a few days after Hepatitis B vaccination.
Methodology: HBsAg test performed on fully automated Chemiluminescence Immunoassay Analyzer (Abbott- Alinity i) using FDA Authorized reagent Kits.
References: Centers for Disease Control
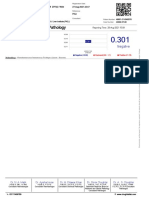
Serum Anti-HCV
Reactive
Cutoff
Value 1.00
Patient
Value 7.05
Non Reactive < 1.0 | Reactive ≥ 1.0
Interpretation:
1)Anti-HCV is a screening test for hepatitis C which detects antibodies to Hepatitis C virus Infection.
2)A reactive Anti-HCV test indicates that the patient has chronic HCV infection.
3)A reactive Anti-HCV test should be followed by HCV RNA testing by PCR.
4)A non-reactive Anti-HCV test indicates that the patient does not have chronic HCV infection and does not warrant further
evaluation.
5)False-negative Anti-HCV test (non-reactive) can be seen in patients with severely immunocompromising conditions, on
hemodialysis, or with suspected acute hepatitis C infection.
Methodology: Anti-HCV test performed on fully automated Chemiluminescence Immunoassay Analyzer (Abbott- Alinity i) using
FDA Authorized reagent Kits.
References: Centers for Disease Control
Dr.Irshad Ansari
Consultant Pathologist
Dr. N. A. Malik Dr. Ayisha Imran Dr. M. Dilawar Khan Dr. Omar Chughtai Dr. A . S. Chughtai
M.B.B.S. (Pb) , M. Phil. M.B.B.S., F.C.P.S. M.B.B.S., M.C.P.S., F.C.P.S. M.B.B.S., M.D., F.C.A.P. M.B.B.S., M.I.A.C., M.Phil.
Consultant Haematologist Consultant Haematologist Consultant Chemical Pathologist Diplomate American Board of Anatomic F.C.P.S., F.C.P.P.Consultant Pathologist
and Clinical Pathology Consultant
Pathologist
03111456789 07 Jail Road Lahore info@chughtailab.com www.chughtailab.com
You might also like
- Pediatric Vaccine Àd Vaccination PDFDocument257 pagesPediatric Vaccine Àd Vaccination PDFntnquynhpro100% (1)
- ANSI HI Version2-2GuideDocument8 pagesANSI HI Version2-2Guideariyamanjula40% (5)
- ANSI HI Version2-2GuideDocument8 pagesANSI HI Version2-2Guideariyamanjula40% (5)
- First Aid CPR AED PDFDocument196 pagesFirst Aid CPR AED PDFwienslaw5804100% (1)
- NP 4Document4 pagesNP 4Hamad RayhanNo ratings yet
- Second Aid USMLE Mnemonics PDFDocument19 pagesSecond Aid USMLE Mnemonics PDFTony Lǎo Hǔ ChenNo ratings yet
- Quick guide to Laboratory Medicine: a student's overviewFrom EverandQuick guide to Laboratory Medicine: a student's overviewNo ratings yet
- MBR CompiledDocument253 pagesMBR CompiledJane GaliciaNo ratings yet
- Microbio Lec 5 - StaphylococcusDocument6 pagesMicrobio Lec 5 - Staphylococcusapi-3743217100% (2)
- RH StudyGuide V2Document32 pagesRH StudyGuide V2NamraNo ratings yet
- BS en Iso 20769-2-2018Document38 pagesBS en Iso 20769-2-2018Khalida MuddasserNo ratings yet
- Ei 1596Document33 pagesEi 1596milecsaNo ratings yet
- Aneamis and HaematologyDocument129 pagesAneamis and HaematologyiwennieNo ratings yet
- Ele. 4 - 130894 - 2020 - 01 - 12-02-53-43 - WatermarkedDocument32 pagesEle. 4 - 130894 - 2020 - 01 - 12-02-53-43 - WatermarkedKhalida Muddasser100% (1)
- QBANK PasTest Best of Fives For DentistryDocument165 pagesQBANK PasTest Best of Fives For DentistrylindajenhaniNo ratings yet
- Ele. 7 - 130891 - 2020 - 01 - 12-03-04-16 - WatermarkedDocument33 pagesEle. 7 - 130891 - 2020 - 01 - 12-03-04-16 - WatermarkedKhalida MuddasserNo ratings yet
- Pourusha VastiDocument54 pagesPourusha Vastiksr prasadNo ratings yet
- Ele. 13 - 210257 - 2020 - 01 - 12-03-53-07 - WatermarkedDocument77 pagesEle. 13 - 210257 - 2020 - 01 - 12-03-53-07 - WatermarkedKhalida MuddasserNo ratings yet
- Ele. 13 - 210257 - 2020 - 01 - 12-03-53-07 - WatermarkedDocument77 pagesEle. 13 - 210257 - 2020 - 01 - 12-03-53-07 - WatermarkedKhalida MuddasserNo ratings yet
- Viral Hepatitis and Blood Glucose ReportsDocument5 pagesViral Hepatitis and Blood Glucose ReportsJunaid Ahmad100% (1)
- ReportViewer AspxDocument2 pagesReportViewer AspxH Shaheen Afzal100% (2)
- Lesson Plan: Mapeh 6Document6 pagesLesson Plan: Mapeh 6Ma. Jasmin Barela100% (2)
- Parasite and Communicable Disease PDFDocument58 pagesParasite and Communicable Disease PDFDr Dhruva PrasadNo ratings yet
- Ele. 12 - 154179 - 2020 - 01 - 12-03-51-20 - WatermarkedDocument61 pagesEle. 12 - 154179 - 2020 - 01 - 12-03-51-20 - WatermarkedKhalida Muddasser100% (1)
- Report ViewerDocument1 pageReport ViewerKoko KhanNo ratings yet
- CLLPatientReport08-31-2022 18 - 02 - 16Document9 pagesCLLPatientReport08-31-2022 18 - 02 - 16Abida Humaira GilaniNo ratings yet
- CLL PatientReportDocument7 pagesCLL PatientReportHassan Sarfraz AliNo ratings yet
- Screenshot 2023-09-03 at 1.27.00 AMDocument8 pagesScreenshot 2023-09-03 at 1.27.00 AMhuzaifaawais27No ratings yet
- CLLPatientReport08-13-2022 17 - 02 - 29Document4 pagesCLLPatientReport08-13-2022 17 - 02 - 29Shahzada irfanNo ratings yet
- Patient Detail: Mrs NafeesaDocument2 pagesPatient Detail: Mrs NafeesaMā H ÂdNo ratings yet
- Covid 19Document1 pageCovid 19Muhammad ali WasimNo ratings yet
- Patient HIV and Urine Test ResultsDocument5 pagesPatient HIV and Urine Test ResultsPsyche's CupidoNo ratings yet
- Patient COVID-19 Antibody Test ResultsDocument1 pagePatient COVID-19 Antibody Test ResultsHibaAliNo ratings yet
- Chughtai Lab ReportDocument3 pagesChughtai Lab Reportjasimhashmi000No ratings yet
- Report ViewerDocument2 pagesReport ViewerDataNo ratings yet
- Patient borderline for Dengue NS1Document1 pagePatient borderline for Dengue NS1Mohammad AliNo ratings yet
- Report 2Document3 pagesReport 2kthandaveswaranNo ratings yet
- Report ViewerDocument1 pageReport ViewerHammad ur Rehman100% (1)
- Screening Report: Department of Pathology Blood Bank ReportDocument1 pageScreening Report: Department of Pathology Blood Bank ReportGhazanfar AliNo ratings yet
- EBM TerapiDocument23 pagesEBM TerapidyahayulestariNo ratings yet
- Report Runita-Kumari Hl6300333762 Hbv-Dna-Quantitative 1710220129Document2 pagesReport Runita-Kumari Hl6300333762 Hbv-Dna-Quantitative 1710220129jhcbokaroNo ratings yet
- Department of Microbiology Urine Examination: Result ResultDocument3 pagesDepartment of Microbiology Urine Examination: Result ResultDanish AmanNo ratings yet
- Manoj Kherajani - 2312122Document2 pagesManoj Kherajani - 2312122dr.menganeNo ratings yet
- CCR 4097XA002626 114023bDocument2 pagesCCR 4097XA002626 114023bsajinbioNo ratings yet
- 4341Document1 page4341Muhammad NaeemNo ratings yet
- Report ViewerDocument1 pageReport Viewerali razaNo ratings yet
- 99 00 11 PDFDocument1 page99 00 11 PDFthqhospitalsanglahillNo ratings yet
- Report ViewerDocument2 pagesReport Viewerjahangirabbas6200No ratings yet
- UnknownDocument3 pagesUnknownNoran RamadanNo ratings yet
- Department of Chemical Pathology: NegativeDocument1 pageDepartment of Chemical Pathology: NegativeWaqas SaleemNo ratings yet
- CLLPatientReport01-21-2024 19 - 27 - 14Document2 pagesCLLPatientReport01-21-2024 19 - 27 - 14Usman EjazNo ratings yet
- Rehman Medical Institute: InterpretationDocument1 pageRehman Medical Institute: InterpretationAbdul MohaiminNo ratings yet
- Results Are To Be Correlated Clinically: Page 1 of 2Document10 pagesResults Are To Be Correlated Clinically: Page 1 of 2rajeshkv1No ratings yet
- Department of Chemical Pathology: Liver Function TestsDocument2 pagesDepartment of Chemical Pathology: Liver Function Testsshahid94lhrNo ratings yet
- Zahida BibiDocument1 pageZahida BibiHabib Ur Rehman BazmiNo ratings yet
- Temar Diagnostics (Head Office) : Clinical PathologyDocument1 pageTemar Diagnostics (Head Office) : Clinical PathologyJaadi 786No ratings yet
- Report ViewerDocument2 pagesReport ViewerHamid RazaNo ratings yet
- Patient Hematology and Serology Lab ResultsDocument1 pagePatient Hematology and Serology Lab ResultsSaba WaheedNo ratings yet
- Blood Test - LFTDocument4 pagesBlood Test - LFTAbhi NikamNo ratings yet
- CLLPatientReport11-08-2021 21 - 46 - 09Document1 pageCLLPatientReport11-08-2021 21 - 46 - 09Kashif JamilNo ratings yet
- 23-Jan-2020 5:15 PM 36 Year(s) /male Brought From Outside 1196 26-Jan-2020 10:12 PM Mashallah Homephty Hospital Mandi YazmanDocument1 page23-Jan-2020 5:15 PM 36 Year(s) /male Brought From Outside 1196 26-Jan-2020 10:12 PM Mashallah Homephty Hospital Mandi YazmanFast ComputersNo ratings yet
- Shalamar Hospital Laboratory: Blood BankDocument2 pagesShalamar Hospital Laboratory: Blood BankSuleman RasheedNo ratings yet
- Ese Linea Brochure Hepatitis Retrovirus m0870003976 e LowDocument4 pagesEse Linea Brochure Hepatitis Retrovirus m0870003976 e LowHadiNo ratings yet
- HbsAg TechincalDocument21 pagesHbsAg Techincalspike9mNo ratings yet
- Herbal DexaDocument8 pagesHerbal DexaAswil NazirNo ratings yet
- Reportviewinpdf - 2022-07-26T191041.034Document1 pageReportviewinpdf - 2022-07-26T191041.034Usman niazNo ratings yet
- Occult HBVDocument5 pagesOccult HBVsoumenNo ratings yet
- Department of Hematology: Coagulation ProfileDocument2 pagesDepartment of Hematology: Coagulation ProfileShaikh Muhammad SaleemNo ratings yet
- Wife Medplus Bills-MergedDocument15 pagesWife Medplus Bills-MergedVamsi Pratap KNo ratings yet
- ITD77731_CPL DiagnosticDocument1 pageITD77731_CPL Diagnosticdhananjay chaudharyNo ratings yet
- Department of Hematology: Blood C/E (Complete, CBC)Document1 pageDepartment of Hematology: Blood C/E (Complete, CBC)Ilyas FaizNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMuhammad YahyaNo ratings yet
- Advanced Medical Laboratory: Ward,, 7853i4833, 768275840, 43067102Document1 pageAdvanced Medical Laboratory: Ward,, 7853i4833, 768275840, 43067102Adil MuradNo ratings yet
- labreportnew (36)Document16 pageslabreportnew (36)mirashaik3No ratings yet
- Department of Chemical Pathology: Serum IronDocument1 pageDepartment of Chemical Pathology: Serum Ironumer azizNo ratings yet
- D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: Name Ref. by Test Asked::: Patientid: Home CollectionDocument2 pagesD-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: Name Ref. by Test Asked::: Patientid: Home Collectionrohit singhNo ratings yet
- Hepatitis and HIV screening reportDocument2 pagesHepatitis and HIV screening reportAftab KhanNo ratings yet
- Hindustan Wellness Lab Report Provides Full Body Wellness ProfileDocument20 pagesHindustan Wellness Lab Report Provides Full Body Wellness ProfileMohammad Ali NPNo ratings yet
- Lab Report - 057196-2122 - 3Document1 pageLab Report - 057196-2122 - 3farhan FHINo ratings yet
- Saleman ReportsDocument1 pageSaleman Reportsazamb5058No ratings yet
- ReportDocument13 pagesReportAbhilashNo ratings yet
- Re-Refined Engine Oil Bottoms (REOB) /vacuum Tower Asphalt Extender (VTAE)Document2 pagesRe-Refined Engine Oil Bottoms (REOB) /vacuum Tower Asphalt Extender (VTAE)Khalida MuddasserNo ratings yet
- Licence VPA10454-025-001 25052018155256Document5 pagesLicence VPA10454-025-001 25052018155256Khalida MuddasserNo ratings yet
- 1.high Level Framework For Process Safety ManagementDocument43 pages1.high Level Framework For Process Safety ManagementKhalida MuddasserNo ratings yet
- Chapter RGDocument95 pagesChapter RGFloresberto MuandaNo ratings yet
- Sanet - ST 9811906599Document246 pagesSanet - ST 9811906599Khalida MuddasserNo ratings yet
- Petroleum BrochureDocument3 pagesPetroleum BrochureKhalida MuddasserNo ratings yet
- 2.technical Workshop Proceedings 2010 - Initial Report Framework High Level Process Safety ManagementDocument41 pages2.technical Workshop Proceedings 2010 - Initial Report Framework High Level Process Safety ManagementKhalida MuddasserNo ratings yet
- GSP-Range-Brochure A4 v2 050916Document12 pagesGSP-Range-Brochure A4 v2 050916Khalida MuddasserNo ratings yet
- Front Matter - 1982 - The Efficient Use of EnergyDocument1 pageFront Matter - 1982 - The Efficient Use of EnergyKhalida MuddasserNo ratings yet
- RCMA BrochureDocument2 pagesRCMA BrochureKhalida MuddasserNo ratings yet
- EI Specifications Qualification Testing and Robustness AssessmentDocument26 pagesEI Specifications Qualification Testing and Robustness AssessmentKhalida MuddasserNo ratings yet
- Fuels Streamlining FR 2020-10-15Document523 pagesFuels Streamlining FR 2020-10-15Khalida MuddasserNo ratings yet
- EB SewerGEMS Personas 1122 FIN LRDocument8 pagesEB SewerGEMS Personas 1122 FIN LRKhalida MuddasserNo ratings yet
- Upgrading Petroleum Residue ReviewDocument14 pagesUpgrading Petroleum Residue ReviewKhalida MuddasserNo ratings yet
- Ele. 2 - 130896 - 2020 - 01 - 12-02-49-07 - WatermarkedDocument36 pagesEle. 2 - 130896 - 2020 - 01 - 12-02-49-07 - WatermarkedKhalida MuddasserNo ratings yet
- Astm D3228-03Document5 pagesAstm D3228-03Marisol ColoradoNo ratings yet
- Upgrading Petroleum Residue ReviewDocument14 pagesUpgrading Petroleum Residue ReviewKhalida MuddasserNo ratings yet
- Pakistan Workshop Promotes Cleaner Fuels, VehiclesDocument29 pagesPakistan Workshop Promotes Cleaner Fuels, VehiclesKhalida MuddasserNo ratings yet
- EU Petrol & DieselUpdateDocument26 pagesEU Petrol & DieselUpdateyazidrasidNo ratings yet
- Larvicidal Activity of Some Saponin Containing Plants Against The Dengue Vector Aedes Aegypti.Document11 pagesLarvicidal Activity of Some Saponin Containing Plants Against The Dengue Vector Aedes Aegypti.Dr. Jawale Chetan S.100% (1)
- Upper Airway Lower Airway: Respiratory ComplicationsDocument6 pagesUpper Airway Lower Airway: Respiratory ComplicationsKhadijah KhanNo ratings yet
- CoccidiaDocument42 pagesCoccidiawadige4668No ratings yet
- ASEPSISDocument41 pagesASEPSISEVRMC ICSNo ratings yet
- A Chilly FeverDocument5 pagesA Chilly FeverChangNo ratings yet
- Henoch Schonlein Purpura (IgA Vasculitis)Document15 pagesHenoch Schonlein Purpura (IgA Vasculitis)Emily Eresuma100% (1)
- Instructions To Disembarking CrewDocument8 pagesInstructions To Disembarking CrewNelfa Dela CruzNo ratings yet
- Family Nursing Care Plans for Environmental RisksDocument7 pagesFamily Nursing Care Plans for Environmental RisksKewkew AzilearNo ratings yet
- Presentation FileDocument25 pagesPresentation FileInnoVentureCommunityNo ratings yet
- Fistula Definition, Causes, Types and TreatmentDocument27 pagesFistula Definition, Causes, Types and TreatmentRaissa Pauline Oliva0% (1)
- Immune System SummaryDocument10 pagesImmune System SummaryLaya ShrbagiNo ratings yet
- Harrison Notes Demo Medicalsupernotes PDFDocument50 pagesHarrison Notes Demo Medicalsupernotes PDFmikeNo ratings yet
- Grade 5 revision for UT4Document10 pagesGrade 5 revision for UT4Fasi HaiderNo ratings yet
- Immunity To TumorDocument38 pagesImmunity To TumorAaryan PatelNo ratings yet
- Press Release - Cholera in KampalaDocument2 pagesPress Release - Cholera in KampalaEmma Laura KisaNo ratings yet
- Candida Species and Oral ThrushDocument11 pagesCandida Species and Oral ThrushHukjvccx JjyfdxxvbNo ratings yet
- Mount Pinatubo InfographicDocument1 pageMount Pinatubo InfographicStephanie SNo ratings yet
- Sepsis and Pneumonia v1.3Document2 pagesSepsis and Pneumonia v1.3Luis HANo ratings yet
- Overview of Covid 19 in Nigeria.Document10 pagesOverview of Covid 19 in Nigeria.KRUSU ISAACNo ratings yet