Professional Documents
Culture Documents
Enzymes Part 1
Uploaded by
Mar LagmayOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Enzymes Part 1
Uploaded by
Mar LagmayCopyright:
Available Formats
ENZYMES Co-Enzymes/Co-Substrates - Derived from dietary vitamins
TYPICAL METAL ION COFACTORS:
GENERAL CHARACTERISTICS OF ENZYMES
Zn2+ (Zinc Ion)
Enzymes – Are catalysts and are not consumed in reactions
Mg2+ (Magnesium Ion)
Proteins that act as a catalyst for Biochemical
Reactions
Mn2+ (Manganese Ion)
Most effective catalysts known
Fe2+ (Ferrous Ion)
Undergo all the reactions of proteins including
NON-METALLIC ION COFACTOR:
DENATURATION
Cl- (Chloride)
Most are GLOBULAR PROTEINS
Inorganic Ion Cofactors - Derived from dietary minerals
Few of them are now known to be RIBONUCLEIC
ACIDS (RNA) NOMENCLATURE AND CLASSIFICATION OF
ENZYMES
The human body has 1000s of this protein Nomenclature - Most commonly named with reference to
Enzyme Activity – Dramatically affected by: their function
Alterations in PH Type of reaction catalyzed
Temperature Identity of the substrate
Substrate – Reactant in an enzyme-catalyzed reaction
Other protein denaturants
Substance upon which the enzyme “acts.”
SIMPLE AND CONJUGATED ENZYMES
THREE IMPORTANT ASPECTS OF THE
TWO TYPES OF ENZYMES NAMING PROCESS
Simple Enzymes 1. Suffix “-ase” identifies it as an enzyme
Conjugated Enzymes EXAMPLES:
Simple Enzyme - Composed only of Protein (AMINO ACID Urease
CHAINS)
Sucrase
Conjugated Enzyme - Has a nonprotein part in addition to a
protein part Lipase
Apoenzyme – Protein part of conjugated enzyme EXCEMPTION – The suffix “-in” is still found in some
Cofactor – Non-protein part of a conjugated enzyme digestive enzymes
Holoenzyme – Biochemically active conjugated enzyme EXAMPLES:
Apoenzyme + Cofactor = Holoenzyme (Conjugated Enzyme) Trypsin
COFACTORS Chymotrypsin
Cofactors - Are important for the chemically reactive
Pepsin
enzymes
Small inorganic molecules 2. Type of reaction catalyzed by an enzyme is often
used as a prefix
Small inorganic ions
EXAMPLES:
Organic Molecule Cofactors – Also called as CO-
Oxidase - Catalyzes an oxidation reaction
ENZYMES or CO-SUBSTRATES
Hyrolase - Catalyzes a hydrolysis reaction Transaminases - Catalyze transfer of an amino
group to a substrate
3. Identity of substrate is often used in addition to
the type of reaction Kinases - Catalyze transfer of a phosphate group
from Adenosine Triphosphate (ATP) to a substrate
EXAMPLE:
HYDROLASE
Glucose Oxidase
Hydrolase - Enzyme that catalyzes a hydrolysis reaction
Pyruvate Carboxylase
Involves addition of a water molecule to a bond to
Succinate Dehydrogenase cause bond breakage
FUNCTION OF FOLLOWING ENZYMES These type of reactions are central to the process of
digestion
Maltase – Hydrolysis of Maltose
Carbohydrases - Hydrolyze glycosidic bonds in oligo- and
Lactate Dehydrogenase - Removal of hydrogen from polysaccharides
lactate ion
Proteases - Effect the breaking of peptide linkages in
Fructose Oxidase - Oxidation of fructose Proteins
Maleate Isomerase - Rearrangement (Isomerization) of Lipases - Effect the breaking of ester linkages in
maleate ion Triacylglycerols
SIX MAJOR CLASSES LYASE
** Enzymes are grouped into SIX MAJOR CLASSES Lyase - Enzyme that catalyzes the addition of a group to a
based on the types of reactions they catalyse ** double bond
Or the removal of a group to form a double bond in a
manner that does not involve hydrolysis or oxidation
Dehydratase - Effects the removal of the components of
water from a double bond
Hydratase - Effects the addition of the components of water
to double bonds
ISOMERASE AND LIGASE
Isomerase - Enzyme that catalyzes the isomerization
(rearrangement of atoms) reactions
Ligase - Enzyme that catalyzes the formation of a bond
OXIDOREDUCTASE between two molecules involving ATP hydrolysis
Oxidoreductase - Catalyzes an oxidation–reduction reaction ATP Hydrolysis - Required because such reactions are
Oxidation and reduction reactions are always linked energetically unfavorable
to one another Require the simultaneous input of energy obtained by
a Hydrolysis of ATP to ADP
Requires a coenzyme that is either oxidized or
reduced as the substrate in the reaction
EXAMPLE:
Lactate Dehydrogenase
TRANSFERASE
Transferase - An enzyme that catalyzes the transfer of a
functional group from one molecule to another
TWO MAJOR SUBSTYPES OF TRANSFERASE:
Induced Fit Model - Substrate contact with enzyme will
change the shape of the active site
Allows small change in space to accommodate
substrate (e.g., how a hand fits into a glove)
A. TRANSFERASE
FORCES THAT DETERMINE SUBSTRATE
B. LYASE
BINDING
ENZYME ACTIVE SITE
H-Bonding
Hydrophobic Interactions
Electrostatic Interactions
ENZYME SPECIFICITY
Absolute Specificity - An enzyme will catalyze a particular
reaction for only one substrate
This is most restrictive of all specificities (not
common)
Urease – Is an enzyme with absolute specificity
Stereochemical Specificity - An enzyme can distinguish
between stereoisomers
Chirality is inherent in an active site (amino acids are
Enzyme Active Site - Relatively small part of an enzyme’s chiral compounds)
structure that is actually involved in catalysis
L-Amino-acid Oxidase - Catalyzes reactions of L-
Place where substrate binds to enzyme amino acids but not of D-amino acids
Formed due to folding and bending of the protein Group Specificity - Involves structurally similar
compounds that have the same functional groups
Usually a “crevice like” location in the enzyme
EXAMPLE:
Some enzymes have more than one active site Carboxypeptidase - Cleaves amino acids one at a time
from the carboxyl end of the peptide chain
Linkage Specificity - Involves a particular type of bond
irrespective of the structural features in the vicinity of the
ENZYME SUBSTRATE COMPLEX bond
Enzyme Substrate Complex - Needed for the activity of Considered most general of enzyme specificities
enzyme EXAMPLE:
Intermediate reaction species formed when substrate Phosphatases - Hydrolyze phosphate–ester bonds in all
binds with the active site types of phosphate esters
Orientation and proximity is favorable and reaction is TEMPERATURE
fast
TWO MODELS FOR SUBSTRATE BINDING TO
ENZYME
Lock and Key Model - Enzyme has a pre-determined shape
for the active site
Only substrate of specific shape can bind with active
site
Optimum pH - pH at which enzyme has maximum
activity
Most enzymes have optimal activity in the pH range
of 7.0 - 7.5
Exception - Digestive enzymes
Pepsin - Optimum pH = 2.0
Trypsin - Optimum pH = 8.0
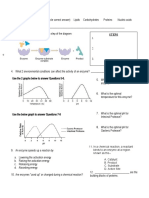
SUBSTRATE CONCENTRATION
Higher temperature results in higher kinetic
energy which causes an increase in number of
reactant collisions, therefore there is higher activity
Optimum Temperature - Temperature at which the
rate of enzyme catalyzed reaction is maximum
Optimum temperature for human enzymes is 37ºC
(body temperature)
Increased temperature (high fever) leads to decreased
enzyme activity Substrate Concentration - At a constant enzyme
concentration, the enzyme activity increases with increased
pH substrate concentration
Substrate Saturation - The concentration at which it reaches
its maximum rate and all of the active sites are full
Turnover Number - Number of substrate molecules
converted to product per second per enzyme molecule under
conditions of optimum Temperature and pH
ENZYME CONCENTRATION
pH changes affect enzyme activity
Drastic changes in pH can result in denaturation of
proteins
Non-Competitive Inhibitors - Do not compete with the
substrate for the same active site
Binds to the enzyme at a location other than active
site
Enzyme Concentration - Enzymes are not consumed in the
reactions they catalyse
At a constant substrate concentration, enzyme
activity increases with increase in enzyme
concentration
The greater the enzyme concentration, the greater the
reaction rate
ANSWERS:
A. Decrease Rate
B. Increase Rate
C. Decrease Rate
D. Decrease Rate
ENZYME INHIBITION
Enzyme Inhibitor – A substance that slows down or stops the
normal catalytic function of an enzyme by binding to it
Competitive Inhibitors - Compete with the substrate for the
same active site
Will have similar charge & shape
You might also like
- A-level Biology Revision: Cheeky Revision ShortcutsFrom EverandA-level Biology Revision: Cheeky Revision ShortcutsRating: 5 out of 5 stars5/5 (5)
- Chem113 LecDocument15 pagesChem113 LecLindon TibleNo ratings yet
- Biochemistry NCMA113 Midterm Notes PDocument3 pagesBiochemistry NCMA113 Midterm Notes PVaishnavi LoganathanNo ratings yet
- Chapter 4 Enzymes and VitaminsDocument9 pagesChapter 4 Enzymes and Vitaminsvictoria cablayNo ratings yet
- Biochemistry Week 3 - EnzymesDocument6 pagesBiochemistry Week 3 - EnzymesMicah JadeNo ratings yet
- EnzymologyDocument13 pagesEnzymologyRane MandapatNo ratings yet
- Dr. Md. Rageeb Md. Usman: Smt. Sharadchandrika Suresh Patil College of Pharmacy, Chopda, Maharashtra, IndiaDocument20 pagesDr. Md. Rageeb Md. Usman: Smt. Sharadchandrika Suresh Patil College of Pharmacy, Chopda, Maharashtra, IndiaAnonymous TCbZigVqNo ratings yet
- Week 9 - EnzymesDocument5 pagesWeek 9 - Enzymesjvlegaspi7463valNo ratings yet
- Enzymes - BiochemistryDocument40 pagesEnzymes - Biochemistrysunil patelNo ratings yet
- 5enzymes and Vitamins PDFDocument48 pages5enzymes and Vitamins PDFRomelyn AngelNo ratings yet
- EnzymesDocument46 pagesEnzymesHighlifeNo ratings yet
- Enzymes and VitaminsDocument19 pagesEnzymes and VitaminsJoanna Marie TulioNo ratings yet
- Enzymes PPTDocument39 pagesEnzymes PPTsunil patelNo ratings yet
- Enzymes PPTDocument40 pagesEnzymes PPTJaisy PatelNo ratings yet
- Enzymes and Vitamins NotesDocument14 pagesEnzymes and Vitamins NotesRealyn ZambasNo ratings yet
- Biochem EnzymesDocument6 pagesBiochem EnzymesvalenciajohannahNo ratings yet
- EnzymesDocument71 pagesEnzymesSaran DNo ratings yet
- Enzymes: Nature, Structure, Classification and Factors Affecting ActivityDocument5 pagesEnzymes: Nature, Structure, Classification and Factors Affecting ActivityFormosa G.No ratings yet
- EnzymesDocument7 pagesEnzymesDANIKA JADE INFIESTONo ratings yet
- Module 4 - Enzymes 1 PDFDocument14 pagesModule 4 - Enzymes 1 PDFFrancis ValdezNo ratings yet
- EnzymeDocument15 pagesEnzymeSheena Mhae LopeñaNo ratings yet
- Bio3110 - Biochemistry Ii-Intermediary Metabolism Enzyme and Enzyme KineticsDocument11 pagesBio3110 - Biochemistry Ii-Intermediary Metabolism Enzyme and Enzyme KineticsNaiomiNo ratings yet
- CC-LEC-NOTES-1Document4 pagesCC-LEC-NOTES-1CDNo ratings yet
- Enzymes: - Sucrase - Lactase - MaltaseDocument4 pagesEnzymes: - Sucrase - Lactase - MaltaseKate Nicole EjercitoNo ratings yet
- Biochem NotesDocument52 pagesBiochem NotesAnonymous 4Ib1ByFC100% (2)
- Biochemistry MidtermDocument16 pagesBiochemistry MidtermKYLENo ratings yet
- Why apoenzymes need cofactorsDocument8 pagesWhy apoenzymes need cofactorsStefanie LucresiaNo ratings yet
- Introduction to Enzymes: The Catalysts of LifeDocument40 pagesIntroduction to Enzymes: The Catalysts of LifeAmal100% (2)
- Btech Great Intro To Enzymes For MLB 104 UseDocument75 pagesBtech Great Intro To Enzymes For MLB 104 Usemaxwell amponsahNo ratings yet
- Động Hoá Học Duoc-32-112Document81 pagesĐộng Hoá Học Duoc-32-112Nguyễn Đình TrườngNo ratings yet
- General Characteristics of Enzyme: Ex: Bread and Alcoholic BeveragesDocument6 pagesGeneral Characteristics of Enzyme: Ex: Bread and Alcoholic BeveragesKyla MacabulosNo ratings yet
- Enzymes. RagaviDocument63 pagesEnzymes. Ragavikavi bharathiNo ratings yet
- Enzym HappyDocument47 pagesEnzym HappyAnggitsb NainggolanNo ratings yet
- C5 - Metabolism and Enzyme Part 1Document20 pagesC5 - Metabolism and Enzyme Part 1Daniel LohNo ratings yet
- CHEM113 Lesson 3 Enzymes and VitaminsDocument6 pagesCHEM113 Lesson 3 Enzymes and VitaminsEli LopezNo ratings yet
- ChemistryDocument7 pagesChemistryLee LuceroNo ratings yet
- Semifinals-Lesson 7-Enzymes and VitaminsDocument4 pagesSemifinals-Lesson 7-Enzymes and Vitaminsino zuii javierNo ratings yet
- 6 EnzymesDocument3 pages6 EnzymesIYA LABAONo ratings yet
- PhmarmacogDocument8 pagesPhmarmacogMichelle CasilangNo ratings yet
- Stocker Enzymes and VitaminsDocument75 pagesStocker Enzymes and VitaminsDylan SalesNo ratings yet
- Introduction to EnzymesDocument79 pagesIntroduction to EnzymesShafaqat Ghani Shafaqat GhaniNo ratings yet
- EnzymesDocument4 pagesEnzymesAstro KeerthanaNo ratings yet
- A. What Are Enzymes?: Enzymes Prepared by La Vera U. Sombito Natural Sciences Department, CAS - USLSDocument4 pagesA. What Are Enzymes?: Enzymes Prepared by La Vera U. Sombito Natural Sciences Department, CAS - USLSJacqueline Rose Alipo-onNo ratings yet
- Biological Molecules EnzymesDocument50 pagesBiological Molecules EnzymesFrance Angel AlbertoNo ratings yet
- Biochem (Finals)Document5 pagesBiochem (Finals)cjyulo99710No ratings yet
- Enzymes PDFDocument36 pagesEnzymes PDFAltamashNo ratings yet
- ENZYMESDocument4 pagesENZYMESLady DanielleNo ratings yet
- Enzymology To Kreb's CycleDocument34 pagesEnzymology To Kreb's Cycleferdinand padillaNo ratings yet
- Lecture 3 - EnzymesDocument19 pagesLecture 3 - EnzymesEiad SamyNo ratings yet
- Nzymes: By: Mrs. Kalaivani Sathish. M. Pharm, Assistant Professor, Pims - PanipatDocument63 pagesNzymes: By: Mrs. Kalaivani Sathish. M. Pharm, Assistant Professor, Pims - Panipaturmila pandeyNo ratings yet
- Enzyme StructureDocument2 pagesEnzyme StructurePrince OycoNo ratings yet
- EnzymesDocument18 pagesEnzymesDRMEHUL DAVE100% (1)
- What Are Enzymes?: EnzymeDocument4 pagesWhat Are Enzymes?: EnzymeMark Jayson ContrerasNo ratings yet
- ENZYME TECHNOLOGY: Biological Catalysts Speed Up Chemical ReactionsDocument131 pagesENZYME TECHNOLOGY: Biological Catalysts Speed Up Chemical ReactionsJanani RipplingrythmNo ratings yet
- OUTLINE8Document8 pagesOUTLINE8Hjm ArNo ratings yet
- 222 Unit 3Document103 pages222 Unit 3Ning BalderasNo ratings yet
- 02 EnzymesDocument63 pages02 EnzymesFatish BanguraNo ratings yet
- CC Lab M1 7Document99 pagesCC Lab M1 7CDNo ratings yet
- EnzymesDocument58 pagesEnzymesFateh RaufNo ratings yet
- Enzymes: Properties, Classification, Clinical SignificanceDocument29 pagesEnzymes: Properties, Classification, Clinical SignificanceWho KnowsNo ratings yet
- PROTEINSDocument5 pagesPROTEINSMar LagmayNo ratings yet
- Lipids Part IiDocument5 pagesLipids Part IiMar LagmayNo ratings yet
- Functional GroupsDocument10 pagesFunctional GroupsMar LagmayNo ratings yet
- Lipids Part IDocument5 pagesLipids Part IMar LagmayNo ratings yet
- Enzymes Part 2Document3 pagesEnzymes Part 2Mar LagmayNo ratings yet
- Cell ReviewerDocument7 pagesCell ReviewerMar LagmayNo ratings yet
- Instructor's Presentation - Epidemiologic Study DesignsDocument96 pagesInstructor's Presentation - Epidemiologic Study DesignsCLAIRE GONONo ratings yet
- CARBOHYDRATESDocument58 pagesCARBOHYDRATESMar LagmayNo ratings yet
- Instructor's Presentation-Screening and SurveillanceDocument49 pagesInstructor's Presentation-Screening and SurveillanceMar LagmayNo ratings yet
- Instructor's Presentation-Causal InferenceDocument19 pagesInstructor's Presentation-Causal InferenceCLAIRE GONONo ratings yet
- Esterifikasi Gliserol Menjadi Triasetin Menggunakan Zeolit AlamDocument7 pagesEsterifikasi Gliserol Menjadi Triasetin Menggunakan Zeolit AlamMhimyMiftahulMawaddahNo ratings yet
- Folio Biology EnzymeDocument10 pagesFolio Biology EnzymeIzZati YazidNo ratings yet
- Enzyme ReviewDocument2 pagesEnzyme ReviewSamura HasibNo ratings yet
- PolymersDocument11 pagesPolymersdovoo lolNo ratings yet
- 2.5 Enzyme Skeleton NotesDocument8 pages2.5 Enzyme Skeleton Notesadri baigorriNo ratings yet
- Biodiesel Production With Green Technologies - Islam e Ravindra (2017) PDFDocument142 pagesBiodiesel Production With Green Technologies - Islam e Ravindra (2017) PDFDiego Bittencourt MachadoNo ratings yet
- Catalase LabDocument7 pagesCatalase LabToga BrandonNo ratings yet
- Catalysis - Petroleum Technology Quarterly - 2014. Refining, Gas Processing and PetrochemicalsDocument68 pagesCatalysis - Petroleum Technology Quarterly - 2014. Refining, Gas Processing and PetrochemicalsjravisrinivasNo ratings yet
- General Organic Chemistry - Iii: Section (A) : Solvents, Reagents and Leaving GroupsDocument20 pagesGeneral Organic Chemistry - Iii: Section (A) : Solvents, Reagents and Leaving GroupsGOURISH AGRAWALNo ratings yet
- Metal Organic Frameworks DissertationDocument10 pagesMetal Organic Frameworks DissertationPaySomeoneToWriteAPaperLafayette100% (1)
- Lindlar MechanismDocument11 pagesLindlar MechanismVo Tung LamNo ratings yet
- STABILITYDocument10 pagesSTABILITYNishamolKSNo ratings yet
- Chemical EngineeringDocument287 pagesChemical EngineeringSleek KastrowNo ratings yet
- Foreword Foreword Foreword ForewordDocument8 pagesForeword Foreword Foreword ForewordShubhamNo ratings yet
- IAEA - Combined Methods For Liquid Radioactive Waste TreatmentDocument250 pagesIAEA - Combined Methods For Liquid Radioactive Waste TreatmentzsuzsapogatsNo ratings yet
- Vu ChemistryDocument52 pagesVu ChemistryShilpendu GhoshNo ratings yet
- Enzyme Inhibition Types & Allosteric RegulationDocument2 pagesEnzyme Inhibition Types & Allosteric RegulationfintastellaNo ratings yet
- Efficient Synthesis of Biodiesel Catalyzed by ChitDocument11 pagesEfficient Synthesis of Biodiesel Catalyzed by ChitRicardo Fajardo DíazNo ratings yet
- Heterogenous Catalyst Joshi, 2015Document10 pagesHeterogenous Catalyst Joshi, 2015Dhinda ClariestaNo ratings yet
- Pressure Drop Modeling SiC FoamsDocument9 pagesPressure Drop Modeling SiC FoamsLykaios Schultz DohrnNo ratings yet
- Mayank Ate PaperDocument19 pagesMayank Ate PaperAbhishekSinghNo ratings yet
- Catalysis Communications: G.O. Ezinkwo, V.F. Tretjakov, R.M. Talyshinky, A.M. Ilolov, T.A. MutomboDocument6 pagesCatalysis Communications: G.O. Ezinkwo, V.F. Tretjakov, R.M. Talyshinky, A.M. Ilolov, T.A. MutomboTalitha AdhyaksantiNo ratings yet
- Plasma HHO To FuelDocument30 pagesPlasma HHO To FuelmikrovoltNo ratings yet
- Extraction of Invertase by Heat Denaturation and Analysis of Invertase Activity by Dinitrosalicylic MethodDocument9 pagesExtraction of Invertase by Heat Denaturation and Analysis of Invertase Activity by Dinitrosalicylic MethodJohn Henrick G. UyNo ratings yet
- 2 - Akbar 2022 - Melamine-Based Porous Network Thin Film at Oil - Water Interface As Effective Catalyst For Reduction of PDocument17 pages2 - Akbar 2022 - Melamine-Based Porous Network Thin Film at Oil - Water Interface As Effective Catalyst For Reduction of Payuk novaNo ratings yet
- Green Synthesis of Palladium Nanoparticles Using Gum Ghatti (Anogeissus Latifolia) and Its Application As An Antioxidant and CatalystDocument10 pagesGreen Synthesis of Palladium Nanoparticles Using Gum Ghatti (Anogeissus Latifolia) and Its Application As An Antioxidant and CatalystSiva PrasadNo ratings yet
- Chem. Rev. 115, 9307-9387 (2015) - Organocatalytic Reactions Enabled by NHCsDocument81 pagesChem. Rev. 115, 9307-9387 (2015) - Organocatalytic Reactions Enabled by NHCssunil_vaman_joshiNo ratings yet
- 14 Charpentier PDFDocument85 pages14 Charpentier PDFKarim Sowley DelgadoNo ratings yet
- Chemical Bandwidth ReportDocument40 pagesChemical Bandwidth ReportterumistarNo ratings yet
- Reactor Design For PetrochemicalDocument40 pagesReactor Design For Petrochemicalsinwei93No ratings yet