Professional Documents
Culture Documents
CPP2 GOC Advan5222948788824536134
Uploaded by
Drushya SalunkeOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CPP2 GOC Advan5222948788824536134
Uploaded by
Drushya SalunkeCopyright:
Available Formats
ORGANIC CHEMISTRY
(JEE-ADVANCED)
GENERAL ORGANIC CHEMISTRY (GOC) CPP-2
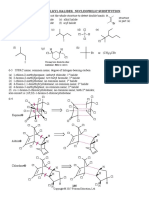
1. Match the column
Column-I Column-II
(Intermediate species)
(I) (II) (III)
(A) Me CH2 Me3C Me2CH (P) I > III > II
(B) Me CH2 Me2CH Me3C (Q) II > III > I
(C) (R) I > II > III
(S) III > I > II
Single Correct :
2. Statement-I : A tertiary carbocation is more stable than a secondary carbocation which is
more stable than a primary carbocation.
Statement-II : The inductive effect operates through -bonds and decrease rapidly with
increase in distance.
(A) Statement-1 is true, statement-2 is true and statement-2 is correct explanation for
statement-1.
(B) Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for
statement-1.
(C) Statement-1 is false, statement-2 is true.
(D) Statement-1 is true, statement-2 is false.
3. Which order is incorrect for stability ?
(A)
CH3–CH2 (B)
Cl Cl Cl
(C) H2C–CH2 > H2C–CH2 > H2C–CH2 (D)
NO2 CN CH3
4. Which order is not correct for stability of resonating structures..
CH2 CH2
(A)
< (B) <
C C
O H O H OCH3 O–CH3
(C) H3C–C=N–CH3 > H3C–CN–CH3 (D)
<
Download ATP STAR APP Page No.1
And Practice For Free
5. Which of the following is correct order of resonance energy?
(A) < (B) >
(C) < (D) >
N
N
H
6. Which of the following is not a valid resonating structure?
H
N
H
CH–CH=CH2
(A) (B)
N
(C) NH3 (D) C
Paragraph for question nos. 7 to 9
Resonance is a kind of interaction between orbitals of a compound, which effects bond
length. Interacting bond orbitals must be in conjugation. The bond length between C2 and
C3 in acryl aldehyde is not equal to the bond length between carbons of ethene because in
acryl aldehyde double bond is in conjugation, so it show the resonance which results the
increase in bond length between C2 and C3 in acryl aldehyde.
CH2=CH–CH = O CH2–CH=CH–O
(3) (2) (1) (3) (2) (1)
Resonance CH CH2 CH2 O
2
Hybrid (3) (2) (1)
7. Which compound will not show the resonance?
NH2
(A) (B) (C) (D)
8. Which compound does not have the conjugative system to show the resonance?
(A) CH2=CH–CC–CH2–CH3 (B)
CH2
(C) (D)
O
9. Find out the correct statement(s) about the given compound?
8 1
7 2
6 3
5 4
(A) Bond length between C2 and C3 = Bond length between C5 and C6
(B) Bond length between C1 and C2 = Bond length between C6 and C7
(C) Bond length between C6 and C7 < Bond length between C3 and C4
(D) Bond length between C2 and C3 < Bond length between C3 and C4
Download ATP STAR APP Page No.2
And Practice For Free
One or more than one correct :
10. Which of the following reaction will form stable ion.
Br
Ph Ph
(A) + aq. AgNO3 (B) + 2K
Ph Ph
Br
O
O
(C) + HBr
(D) HBF4
OH
11. Which of the following are incorrect.
(A) Order of heat of hydrogenation C–C=C–C–C–C=C–C < C–C–C=C–C=C–C–C
(B) Order of heat of combustion > > >
(C) Order of stability of carbocation > >
N N N
(D) Order of heat of combustion cis-1,4-bis-(t-butyl) cyclohexane<Trans-1,4-bis-(t-butyl)
cyclohexane
Match the column
12. Column-I Column-II
(A) (P) Planar
(B) (Q) Non-planar
N
(C) (R) (4n + 2) electron
(D) (S) (4n) electron
Paragraph for Question Nos. 13 to 15
Aromatic compound are these that meet the following criteria :
(i) The structure must be cyclic, containing (4n + 2) electrons
(ii)Each atom in the ring must have unhybridized p-orbitals
(iii) Unhybridized p-orbitalmust overlap orbitals.
(iv) Structure must be planar
In anti-aromatic compound (4n) electron are delocalization, but delocalization of pi electrons
over the ring increases the electronic energy.
13. Which of following compound is aromatic?
H H
N N H–N N H–N N–H H–N N–H
H H
(A) (B) (C) (D)
14. Which of following is anti aromatic?
(A) (B) (C) (D) All
O
Download ATP STAR APP Page No.3
And Practice For Free
15. In which of following reaction product formed is aromatic?
OH
OH
H H KH
(A) (B) (C) (D) All
16. Which of the following is the correct order of bond energy for indicated C=C bonds?
(x) (y) (z)
(A) x > y > z (B) z > y > x (C) z > x > y (D) None of these
Paragraph for question nos. 17 & 18
d
OH
O2N NO2
e c
H2N COOH
NO2
f b
CH3–HN SO3H
a
HO
NH–C–CH3
g
O
17. How many moles of NaHCO3 will consume on reaction with 1 mole of this compound?
(A) 2 (B) 3 (C) 4 (D) 1
18. What is the correct order of basic strength of the following?
(A) e > f > g (B) f > e > g (C) g > e > f (D) e > g > f
19. Correct order of decreasing basic strength of the given compound is.
(I) (II) Et–N–Et (III) (IV)
N Et N N
(A) IV > III > II > I (B) I > II > III > IV (C) IV > III > I > II (D) I > II > IV > III
One or more than one correct :
20. Select correct options.
NH2 NH2
Me
(A) basicity (B) < basicity
<
N N
COOH COOH OH OH
Me
(C) < acidity (D) < acidity
ANSWER KEY
1. A Q; B R; C P 2. (A) 3. (C) 4. (C)
5. (D) 6. (C) 7. (B) 8. (D) 9. (B)
10. (ABD) 11. (ABCD) 12. (A) P, R; (B) P, R; (C) Q,R; (D) Q,S
13. (C) 14. (A) 15. (D) 16. (C) 17. (A)
18. (B) 19. (D) 20. (BCD)
Download ATP STAR APP Page No.4
And Practice For Free
You might also like
- Advanced Organic Chemistry by David LewisDocument1,176 pagesAdvanced Organic Chemistry by David LewisRoque Valeroso Cuellar100% (1)
- Sample Acs Final ExamDocument27 pagesSample Acs Final Examjilo100% (2)
- 2.11 MechanismDocument38 pages2.11 MechanismAmber Michaels100% (1)
- Goc + IsomerismDocument5 pagesGoc + IsomerismRohail HussainNo ratings yet
- PACE Final Lap (Organic Chemistry) PDFDocument152 pagesPACE Final Lap (Organic Chemistry) PDFAman AdatiaNo ratings yet
- Goc Stereo PDFDocument32 pagesGoc Stereo PDFDeepak GargNo ratings yet
- Chapter 7 PDFDocument80 pagesChapter 7 PDFBaban BaidyaNo ratings yet
- Understanding Mass SpectrometryDocument147 pagesUnderstanding Mass SpectrometryYee Kin WengNo ratings yet
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- GOC DPPDocument35 pagesGOC DPPRayNo ratings yet
- CPP (GOC-2) RBooster ResDocument10 pagesCPP (GOC-2) RBooster RessahilNo ratings yet
- First Year - GOC, ISOMERISM - Revision - CPP - CKHDocument3 pagesFirst Year - GOC, ISOMERISM - Revision - CPP - CKHSeaN GabrielNo ratings yet
- Gocdpp 60Document8 pagesGocdpp 60Mrigank GuptaNo ratings yet
- Allen DPP1 Hydrocarbons PDFDocument6 pagesAllen DPP1 Hydrocarbons PDFJannaki PvNo ratings yet
- Alkyl Halide: Organic ChemistryDocument56 pagesAlkyl Halide: Organic ChemistryAaryan KeshanNo ratings yet
- DPP GoccccDocument10 pagesDPP GoccccMayur Khichi0% (1)
- General Organic Chemistry Assignment SolutionsDocument7 pagesGeneral Organic Chemistry Assignment Solutionsrahul kmNo ratings yet
- Day - 28 Goc-2 (27-12-2022) ChemistryDocument4 pagesDay - 28 Goc-2 (27-12-2022) ChemistryVIKRANTH KUMAR JAKKOJUNo ratings yet
- Goc and Isomerism d95DOpg9PVCoDaM2Document51 pagesGoc and Isomerism d95DOpg9PVCoDaM2moviea849No ratings yet
- Oc PT 2 - Student Copy - (Eng)Document6 pagesOc PT 2 - Student Copy - (Eng)Ramkumar SundaramNo ratings yet
- NEET Organic Chemistry - Some Basic Principles and Techniques Important QuestionsDocument18 pagesNEET Organic Chemistry - Some Basic Principles and Techniques Important QuestionsThamizharuvi. ANo ratings yet
- GocDocument20 pagesGocSuyog SardaNo ratings yet
- 2024_03_10_JM_25A_ONLINE.pmd_1328930_2024_03_12_09_16Document17 pages2024_03_10_JM_25A_ONLINE.pmd_1328930_2024_03_12_09_16gakot74126No ratings yet
- Final 100 Question Bank for JEE Main 2020 ChemistryDocument29 pagesFinal 100 Question Bank for JEE Main 2020 Chemistryv k (venkat da)No ratings yet
- JEE ORGANIC CHEMISTRY REVIEWDocument5 pagesJEE ORGANIC CHEMISTRY REVIEWDrushya SalunkeNo ratings yet
- Alkene DPPDocument20 pagesAlkene DPPKalyan ReddtNo ratings yet
- DPP 3 Electronic EffectDocument3 pagesDPP 3 Electronic EffectUsha SherkhaneNo ratings yet
- Goc 6002eec9ca05fDocument14 pagesGoc 6002eec9ca05fAyush TiwariNo ratings yet
- Mixed DPP 8 - 20 (Isomerism)Document37 pagesMixed DPP 8 - 20 (Isomerism)shresthgaur19No ratings yet
- Goc Sheet-3-EfDocument6 pagesGoc Sheet-3-EfAaRaV KuShWaHaNo ratings yet
- DPP 2 Structural Id & POC-1 VKP Sir-3697Document2 pagesDPP 2 Structural Id & POC-1 VKP Sir-3697Parth KadamNo ratings yet
- Vibrant Academy: (India) Private LimitedDocument6 pagesVibrant Academy: (India) Private LimitedRk ChaudharyNo ratings yet
- Exercise - IV: (One or More Than One Option Correct)Document3 pagesExercise - IV: (One or More Than One Option Correct)Anurag KumarNo ratings yet
- Bakliwal Tutorials: Topic: Inductive Effect Part - A: SubjectiveDocument3 pagesBakliwal Tutorials: Topic: Inductive Effect Part - A: SubjectiveRitesh SonawaneNo ratings yet
- Physical Properties & POC ExerciseDocument15 pagesPhysical Properties & POC ExercisergNo ratings yet
- Physical Properties & POC ExerciseDocument15 pagesPhysical Properties & POC ExercisergNo ratings yet
- Classification & Nomenclature of OC - Problem Solving - JEE Sheet PDFDocument16 pagesClassification & Nomenclature of OC - Problem Solving - JEE Sheet PDFShadowNo ratings yet
- BR (D) BR (C) : (A) I II III IV (B) IV III II I (C) IV III I II (D) I II IV IIIDocument2 pagesBR (D) BR (C) : (A) I II III IV (B) IV III II I (C) IV III I II (D) I II IV IIIHemendra PrasannaNo ratings yet
- Chemistry QuestionDocument5 pagesChemistry QuestionPrithviraj GhoshNo ratings yet
- Goc-II Ex e SiamrpnDocument22 pagesGoc-II Ex e SiamrpnArchit KhemaniNo ratings yet
- General Organic Chemistry(GOC) _ DPP 01_removedDocument2 pagesGeneral Organic Chemistry(GOC) _ DPP 01_removedshishiranand25No ratings yet
- DOCUMENT CHEMISTRY MULTIPLE CHOICE QUESTIONSDocument8 pagesDOCUMENT CHEMISTRY MULTIPLE CHOICE QUESTIONSJAIMIN PATELNo ratings yet
- Reactive Intermediate TestDocument6 pagesReactive Intermediate TestDhruv patelNo ratings yet
- Set of 50 Obj in General Organic Chemistry by S.K.sinha HTTP://WWW - Openchemistry.inDocument6 pagesSet of 50 Obj in General Organic Chemistry by S.K.sinha HTTP://WWW - Openchemistry.inmyiitchemistry50% (4)
- DPP # 09 TIME: 30 Min.: 1. Column I Column II (Compounds) (Properties)Document2 pagesDPP # 09 TIME: 30 Min.: 1. Column I Column II (Compounds) (Properties)ADARSH KUMAR BEHERANo ratings yet
- IsomerismDocument62 pagesIsomerismanuragrana12345678No ratings yet
- Worksheet 1Document16 pagesWorksheet 1All photos CloudNo ratings yet
- ExerciseDocument50 pagesExerciseAbhiNo ratings yet
- Organic Chemistry: Daily Practice ProblemsDocument53 pagesOrganic Chemistry: Daily Practice ProblemsRaju SinghNo ratings yet
- Narayana Iit Academy Narayana Iit AcademDocument57 pagesNarayana Iit Academy Narayana Iit AcademDeepak PhogatNo ratings yet
- Quiz-General Organic Chemistry & Isomerism-Snd - SNDDocument4 pagesQuiz-General Organic Chemistry & Isomerism-Snd - SNDayesha sheikhNo ratings yet
- 23 Feb CDE GOC1 - TwoDocument21 pages23 Feb CDE GOC1 - TwoShreyaNo ratings yet
- Nomenclature - Problem Solving Neet TSC PDFDocument16 pagesNomenclature - Problem Solving Neet TSC PDFRishikesh SinghNo ratings yet
- Goc 1Document3 pagesGoc 1Twisha ViraniNo ratings yet
- General Organic Chemistry - DPP 01Document3 pagesGeneral Organic Chemistry - DPP 01suryawanshiashish549No ratings yet
- Part - I: Subjective Questions: Section (A) : Electrophile, Nucleophile, Nucleophilicity, Leaving Group Ability SolventDocument18 pagesPart - I: Subjective Questions: Section (A) : Electrophile, Nucleophile, Nucleophilicity, Leaving Group Ability SolventRavi kumarNo ratings yet
- GOC document summarizes key organic chemistry conceptsDocument3 pagesGOC document summarizes key organic chemistry conceptsVanshdip RawatNo ratings yet
- DPP 4-7 (Isomerism)Document18 pagesDPP 4-7 (Isomerism)socialbeing006No ratings yet
- GOC Sheet PDFDocument55 pagesGOC Sheet PDFAayush KharbandaNo ratings yet
- Booklet On GOC - 1 (FC)Document8 pagesBooklet On GOC - 1 (FC)Harshvardhan SinghNo ratings yet
- Solution Iit JeeDocument65 pagesSolution Iit Jeebishuu100% (1)
- Narayana Solutions Iit Jee 2010Document57 pagesNarayana Solutions Iit Jee 2010Ashish Kumar0% (1)
- INORGANIC S-BLOCKDocument4 pagesINORGANIC S-BLOCKDrushya SalunkeNo ratings yet
- JEE ORGANIC CHEMISTRY REVIEWDocument5 pagesJEE ORGANIC CHEMISTRY REVIEWDrushya SalunkeNo ratings yet
- S-Block Elements OverviewDocument13 pagesS-Block Elements OverviewDrushya SalunkeNo ratings yet
- Complete S Block ElementsDocument108 pagesComplete S Block ElementsDrushya SalunkeNo ratings yet
- Goc and Isomerism Notes - PMDDocument46 pagesGoc and Isomerism Notes - PMDPritesh KakaniNo ratings yet
- RearrangementsDocument115 pagesRearrangementsscadvijayNo ratings yet
- ACS Review 10 Conjugation in Alkadienes and Allylic SystemsDocument16 pagesACS Review 10 Conjugation in Alkadienes and Allylic SystemsRima MghamesNo ratings yet
- Chapter 6-Alkyl Halides Nucleophilic Substitution: You May Have Drawn The Other Enantiomer. Either Is CorrectDocument23 pagesChapter 6-Alkyl Halides Nucleophilic Substitution: You May Have Drawn The Other Enantiomer. Either Is Correct張湧浩No ratings yet
- 191 Reactions Producing StereoisomersDocument3 pages191 Reactions Producing StereoisomersM DiNo ratings yet
- Fundamentals of Rganic Chemistry Note For Year I PharmacyDocument151 pagesFundamentals of Rganic Chemistry Note For Year I PharmacySelam 1No ratings yet
- Six Pillars of Organic Chemistry: (1) - The Objectives of The Six Pillar Approach Are (I) BuildingDocument5 pagesSix Pillars of Organic Chemistry: (1) - The Objectives of The Six Pillar Approach Are (I) BuildingVictor GuillénNo ratings yet
- Non Classical CarbocationsDocument55 pagesNon Classical CarbocationsMungara Srinivas50% (4)
- Reactions of Alkenes and Alkynes Study GuideDocument17 pagesReactions of Alkenes and Alkynes Study GuideMelissa GarciaNo ratings yet
- Halogenoalkane ReactivityDocument16 pagesHalogenoalkane Reactivitydiyaray100% (1)
- GOC NotesDocument43 pagesGOC NotesSudhanshu HedaNo ratings yet
- Haloalkane & Haloarene CBSE PYQDocument13 pagesHaloalkane & Haloarene CBSE PYQRithvik Kumar100% (1)
- OxfordAQA 9620 CH02 WRE Jun22 v1.0Document5 pagesOxfordAQA 9620 CH02 WRE Jun22 v1.0MirandaxxNo ratings yet
- Chapter 6 Hydrocarbons and Alkenes SolutionsDocument26 pagesChapter 6 Hydrocarbons and Alkenes SolutionsjanNo ratings yet
- Olah1995 Noble LectureDocument13 pagesOlah1995 Noble LectureRohan TiwariNo ratings yet
- Lesson Plan 8Document3 pagesLesson Plan 8Gagan DeepNo ratings yet
- Lab Report 10 Friedel CraftsDocument7 pagesLab Report 10 Friedel CraftsPancho Abarca ZuñigaNo ratings yet
- Mark Scheme Synthesis and Analytical TechniquesDocument33 pagesMark Scheme Synthesis and Analytical TechniquesAddan AddanNo ratings yet
- New CHY3201 Chapter 9 Addition ReactionDocument31 pagesNew CHY3201 Chapter 9 Addition Reaction222418No ratings yet
- Unit 2 L1 523 2022Document41 pagesUnit 2 L1 523 2022Rupam MondalNo ratings yet
- PDFDocument91 pagesPDFSatyam KumarNo ratings yet
- Unusual Optical Activity 2019Document62 pagesUnusual Optical Activity 2019Ankita KumariNo ratings yet
- An Incoming Group Replaces A Leaving Group: Substitution ReactionsDocument2 pagesAn Incoming Group Replaces A Leaving Group: Substitution Reactionsbabybri94No ratings yet
- 3.3.4 Alkenes FullDocument39 pages3.3.4 Alkenes FulllfcluishoughtonNo ratings yet
- Organic Chemistry-I Reactive Intermeditate - Carbonium Ions OnlyDocument12 pagesOrganic Chemistry-I Reactive Intermeditate - Carbonium Ions Onlyboopathi_chemist3628No ratings yet
- Wrong Answered Questions: You MarkedDocument13 pagesWrong Answered Questions: You MarkedKhelan MehtaNo ratings yet