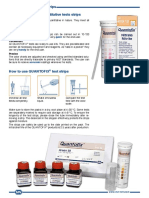
Professional Documents
Culture Documents
BIOMERIEUX BIOBALL Redefining Quantitative Microbiological Quality Contr...
Uploaded by
Fadhlan ArifinOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
BIOMERIEUX BIOBALL Redefining Quantitative Microbiological Quality Contr...
Uploaded by
Fadhlan ArifinCopyright:
Available Formats
Redefining Quantitative Microbiological Quality
Control: A review of released BIOBALL® MultiShot
550 batches from 2014 to 2018
BRENDAN TINDALL
INTRODUCTION PRODUCT TESTING
Unlike our counterparts in the chemical world, consistently precise and accurate results The mean and standard deviation of each batch is determined by a minimum of 50
from microbiological quality control are encountered less often. In other words, quantitative counts(i.e. aliquots counted on a singular plate of the relevant agar type) per batch
Microbiological testing allows for (fairly large) discrepancies in the results due to the following internal standard operating procedures. The mean CFU per Bioball target is
inherent difficulties in dealing with living organisms as well as quantifying them. The 550±50 CFU for the MultiShot 550 product and the standard deviation target is ≤10% the
intellectual properties and proprietary methodologies used in making BIOBALL has product mean.
redefined what was considered as Quantitative Microbiology and has made it simple to get
precise & accurate quantitative microbiological quality controls batch to batch. BIOBALL The standard operating procedure for the quality control plating of the various batch mimics
was developed in Sydney Australia in 2001 and the business was purchased by bioMerieux the “Instructions for Use” (detailed in the relevant instruction manual sent with each
in 2007. The product continues to be manufactured in Sydney Australia and is now product). All plating and counting is done manually with dedicated and experience staff.
available for purchase all over the world, with millions of tests conducted every year. Details of the agar plates used and incubation conditions for each organism are detailed in
Table 1.
SUMMARY Table 1: List of the different organisms offered in the 550 format
Recommended Agar Recommended Cell culture Number of released
Organism
The internal Quality Control results of BIOBALL MultiShot 550 batches released from the Culture media Incubation conditions preparation Type batches (2014-2018)
Aspergillus brasiliensis DRBC agar 37 °C Spore 83
beginning of 2014 to 2018 were compiled in this review, all results summarized herein were Bacillus subtilis Nutrient agar 37 °C Spore 87
well within the established specifications. The results from accelerated stability testing and Burkholderia cepacia Nutrient agar 37 °C Cell 18
annual real time testing at 13 and 25 months were also included. These results indicate Candida albicans Nutrient agar 37 °C Cell 90
that a mean of 45 – 55 CFU per aliquot could be maintained for all investigated batches to Pseudomonas aeruginosa Nutrient agar 37 °C Cell 98
the end of expected shelf life. Staphylococcus aureus Nutrient agar 37 °C Cell 96
PRODUCT INTRODUCTION RESULTS – QUALITY CONTROL RESULT
The BIOBALL product is a small freeze dried water soluble ball containing a precise Figure 1 through 6 illustrate the quality control results of all the released batches for the 6
number of viable microorganisms. The manufacturing process uses patented technology investigated strains. It can be seen that all the means are well within the expected range.
and proprietary techniques for precision and viability. A flow cytometer is used to select Further sections will analyse the batches stability and moving range.
individual cells from a culture and dispense them into a single droplet. Each droplet
therefore contains a precise number of viable cells that have been individually selected for
maximum recovery rate on non-selective media. This technology is capable of
distinguishing between single cells and cell clusters in cultures such as Staphylococcus
aureus. Droplets are frozen in liquid nitrogen, dispensed in vials, freeze-dried and sealed
under vacuum for maximum shelf-life and recovery.
BIOBALL is manufactured in Sydney, Australia and produced to the world’s highest quality
standards, based on ISO:17034 accreditation. The BIOBALL manufacturing site, which is
accredited as a Reference Materials producer, was the first company accredited for
quantitative microbiological reference standards in the world. ISO:17034 accreditation is
issued by NATA, Australia’s government endorsed provider of accreditation for laboratories
and similar testing facilities. In 2018 BIOBALL available strains products have all been
accredited as “Certified Reference Materials”.
The BIOBALL comes in four distinct quantitative ranges, SingleShot, MultiShot 550,
HighDose 10K and MultiShot 10e8. The SingleShot product is designed for single use. The
MultiShot 550 is designed specifically to optimise growth promotion testing workflows. The
550 product is designed to dispense 10, 100μL doses of 50 CFU with minimal variance
between results. The HighDose product is generally used for large high-dose inoculations.
Finally, the MultiShot 10e8 product is designed for Antimicrobial/Preservative Effectiveness
testing.
Due to the unique manufacturing process for BIOBALL every batch has a specific target
mean CFU number (not range) and has very tight quality control release requirements. Figure 1: Summary graph of the quality control results of the released Bioball 550
Graphing of the last four years of MultiShot 550 batches shows minimal batch-to-batch batches of Aspergillus brasiliensis: error bars represent standard deviation based
variability with respect to time. Each batch of BIOBALL listed below was recovered on on the repeated aliquots; Red lines indicate accepted mean range
different nutrient agar batches strongly suggesting the results are independent of media
batches.
MICROORGANISMS
The BIOBALL MultiShot 550 has 11 microorganisms in its range including bacteria, yeast
and mould. These include the 5 major strains for GPT in Microbial Enumeration Tests USP
<61> and 2.6.12: Aspergillus brasiliensis, Bacillus subtilis, Candida albicans,
Pseudomonas aeruginosa and Staphylococcus aureus. As well as the additional strains
from USP <71> and 2.6.13 test for Specified Microorganisms: Clostridium sporogenes,
Escherichia coli and Salmonella abony. Enterococcus faecalis and Propionibacterium
acnes were added to the product line per market request. Finally included is Burkholderia
cepacia which currently is not required in any pharmacopoeias yet but is included in the
Latest USP revisions from the USP Microbiology expert committee. Also the FDA has
stated that they will not release any new products without specific Burkholderia cepacia
testing. (https://www.fda.gov/Drugs/DrugSafety/ucm559508.htm)
This study will focus on the results from the 5 major strain for GTP (Aspergillus brasiliensis,
Bacillus subtilis, Candida albicans, Pseudomonas aeruginosa and Staphylococcus aureus)
plus the Burkholderia cepacia strain.
Figure 2: Summary graph of the quality control results of the released Bioball 550
batches of Bacillus subtilis: error bars represent standard deviation based on the
repeated aliquots; Red lines indicate accepted mean range
Page 1 on 2 – Version 1 – January 2019
Redefining Quantitative Microbiological Quality
Control: A review of released BIOBALL® MultiShot
550 batches from 2014 to 2018
BRENDAN TINDALL
SUMMARY
Figure 3:Summary graph of the quality control results of the released Bioball 550
batches of Burkholderia cepacia: error bars represent standard deviation based on
the repeated aliquots; Red lines indicate accepted mean range Figure 6: Summary graph of the quality control results of the released Bioball 550
batches of Staphylococcus aureus: error bars represent standard deviation based
on the repeated aliquots; Red lines indicate accepted mean range
RESULTS – BATCH STABILITY (13 AND 25 MONTHS)
As per internal procedure, a small selection of the released batches are tested for stability
at mid and end of shelf life time. Table 2 shows a summary of this testing per species. It
can be attested that all tested batches remained stable over 25 months (13 and 25 months
average cfu within 10% of the original average measured cfu).
Table 2: Summary of long term stability result per investigated species
Organism Original CFU- Mean CFU 13 months - Mean CFU 25 months - Mean n
Aspergillus brasiliensis 49.9 46.2 48.1 3
Bacillus subtilis 47.0 46.3 47.4 3
Burkholderia cepacia 51.2 49.3 49.3 9
Candida albicans 49.0 44.8 46.3 8
Pseudomonas aeruginosa 49.6 45.9 46.9 6
Staphylococcus aureus 50.8 50.3 50.6 4
RESULTS – MOVING RANGE
Figure 4: Summary graph of the quality control results of the released Bioball 550
batches of Candida albicans: error bars represent standard deviation based on the The moving range is a useful tool to monitor process variation. The moving range is defined
repeated aliquots; Red lines indicate accepted mean range; Red lines indicate as the difference between data point and its predecessor. In our case, it would be the
accepted mean range difference in measured cfu mean from a batch and its predecessor. This measurement can
help quality control personnel maintain an eye on short term bursts of variation and
possible longer term trends. Figure 7 summarizes the moving range charts for each
species. It can be assessed that the moving ranges are quite low for all the different
Multishsot product presented here (average less than 3 cfu and maximum upper control
limit (UCL) of 6.7 cfu).
Figure 5: Summary graph of the quality control results of the released Bioball 550
batches of Pseudomonas aeruginosa: error bars represent standard deviation based Figure 7: Moving range charts for all investigated species: a) Aspergillus
on the repeated aliquots; Red lines indicate accepted mean range brasiliensis, b) Bacillus subtilis, c) Burkholderia cepacia, d) Candida albicans, e)
Pseudomonas aeruginosa and f) Staphylococcus aureus
CONCLUSION
The data presented in this review reveals that all released batches from 2014 to 2018,
across all commercialized organisms, are well within established specifications and show
high level of reproducibility over time (average moving range of less than 3 cfu across all
organisms). All real time stability results are also within specifications (13 and 25 months
average cfu within 10% of the original average measured cfu). Based on the presented
results, we could infer that, notwithstanding major changes in internal procedures or major
deviations, the accumulated expertise regarding the 550s will continue to yield a reliably
Page 2 on 2 – Version 1 – January 2019 accurate and precise product.
You might also like
- 51 PDFDocument3 pages51 PDFChetalee NaikNo ratings yet
- SHP Laboklav Manual PDFDocument35 pagesSHP Laboklav Manual PDFAntoni Gandia100% (1)
- Alternative Methods for Real-Time Microbiological Quality ControlDocument10 pagesAlternative Methods for Real-Time Microbiological Quality ControlLEPESANTNo ratings yet
- Guidance Manual: Quality Control Testing of Human AlbuminDocument35 pagesGuidance Manual: Quality Control Testing of Human AlbuminNataraja Sekhar Reddy VaddamaniNo ratings yet
- BME-01 Equipment Iddentification TagDocument4 pagesBME-01 Equipment Iddentification TagPAPPU RANJITH KUMARNo ratings yet
- WP Gmp-En AnshDocument12 pagesWP Gmp-En AnshFelix ShihNo ratings yet
- CalibrationDocument5 pagesCalibrationMonika KshNo ratings yet
- Environmental Monitoring Risk AssessmentDocument22 pagesEnvironmental Monitoring Risk AssessmentMarcelo CarvalhoNo ratings yet
- IPQA A Beginner's GuideDocument170 pagesIPQA A Beginner's GuideGoran MickoNo ratings yet
- ObjectionableDocument9 pagesObjectionabledmtalbhogeNo ratings yet
- MilliQ WaterDocument16 pagesMilliQ Wateralen19819072No ratings yet
- Validation of Microbial Recovery From Pharmacopeial Articles EpaDocument10 pagesValidation of Microbial Recovery From Pharmacopeial Articles EpaMarco HernandezNo ratings yet
- Application of Poisson Distribution in Establishing Control Limits for Discrete Quality AttributesDocument10 pagesApplication of Poisson Distribution in Establishing Control Limits for Discrete Quality AttributesJavier GalvanNo ratings yet
- HoldTime 01Document4 pagesHoldTime 01Anonymous GPLMks1pNo ratings yet
- 41 (1) In-Process Revision - 1790 - Visual Inspection of InjectionsDocument10 pages41 (1) In-Process Revision - 1790 - Visual Inspection of InjectionsBudy WijiyantoNo ratings yet
- Container Closure Integrity of Sterile Pharmaceutical Containers - Richard MilletDocument36 pagesContainer Closure Integrity of Sterile Pharmaceutical Containers - Richard MilletmsorianolNo ratings yet
- G OwningDocument6 pagesG Owningk.p.100% (1)
- Protocol PQDX 241 v4 Capillary BloodDocument18 pagesProtocol PQDX 241 v4 Capillary Bloodludi100% (1)
- 2017 11 22 Guidelines GMP For AtmpsDocument90 pages2017 11 22 Guidelines GMP For Atmpserdo mandanaNo ratings yet
- Oq Faw1005Document66 pagesOq Faw1005vijayns_250355172No ratings yet
- Whythe10 ppmCriterionShouldBeAbandonedDocument5 pagesWhythe10 ppmCriterionShouldBeAbandonedMuhammad AsifNo ratings yet
- MICRO 4 SOP For Microbial Monitoring in Drain Point of Pharmaceutical Manufacturing SitesDocument2 pagesMICRO 4 SOP For Microbial Monitoring in Drain Point of Pharmaceutical Manufacturing SitesAjesh Tk100% (1)
- Sop For Bio-BurdenDocument5 pagesSop For Bio-BurdenMusyoka UrbanusNo ratings yet
- Final Microbiology Method Guidance 110409.Pdf11Document107 pagesFinal Microbiology Method Guidance 110409.Pdf11rominutza23No ratings yet
- Validation of Sterilization: GMP and Qa Class: BDocument50 pagesValidation of Sterilization: GMP and Qa Class: BHikmah Purnama AzaniNo ratings yet
- Eugon LT 100 BrothDocument2 pagesEugon LT 100 BrothSergei VoychukNo ratings yet
- Bioburden USP PDFDocument4 pagesBioburden USP PDFKatyaSNNo ratings yet
- GLP Study PlanDocument45 pagesGLP Study PlanashishmathewNo ratings yet
- T309 - Microbiological Quality Control Requirements in An ISO 17025 Laboratory PDFDocument26 pagesT309 - Microbiological Quality Control Requirements in An ISO 17025 Laboratory PDFПламен ЛеновNo ratings yet
- WHO Expert Committee On Specifications For Pharmaceutical PreparationsDocument324 pagesWHO Expert Committee On Specifications For Pharmaceutical PreparationsKulfi BarfiNo ratings yet
- Cleaning and disinfection of food factories: a practical guideFrom EverandCleaning and disinfection of food factories: a practical guideNo ratings yet
- 3 2 - MicrobiologicalQualityControl 1Document37 pages3 2 - MicrobiologicalQualityControl 1Tong ChanNo ratings yet
- An Inside Look at USP71Document22 pagesAn Inside Look at USP71Dante IulliNo ratings yet
- Blood Bags (JTP)Document7 pagesBlood Bags (JTP)arthisoundaryaNo ratings yet
- Autoclave Efficacy Testing Procedure - 0 PDFDocument2 pagesAutoclave Efficacy Testing Procedure - 0 PDFAnjali YadavNo ratings yet
- Transport of CellsDocument20 pagesTransport of CellsshneetsNo ratings yet
- Microbiology Product Catalog EU enDocument94 pagesMicrobiology Product Catalog EU enArifin R HidayatNo ratings yet
- Validation of Methods of Analysis Application in Food MicrobiologyDocument36 pagesValidation of Methods of Analysis Application in Food MicrobiologynilayNo ratings yet
- Knapp Test PDFDocument2 pagesKnapp Test PDFAziz Aditya WigunaNo ratings yet
- Meclizine HCLDocument10 pagesMeclizine HCLChEng_No ratings yet
- Balanza Precisa XB220ADocument73 pagesBalanza Precisa XB220ATincho ValleverdeNo ratings yet
- Friabilator Operation Cleaning Handling Standard Operating ProcedureDocument8 pagesFriabilator Operation Cleaning Handling Standard Operating ProcedureKumar Galipelly100% (1)
- 320 Other 1210 2 10 20180111Document10 pages320 Other 1210 2 10 20180111Vivek PrasadNo ratings yet
- Justification of LimitsDocument6 pagesJustification of LimitsRulli SulaemanNo ratings yet
- Validation Protocol Shows Sterilization Process RetrainingDocument43 pagesValidation Protocol Shows Sterilization Process RetrainingSalome QarchavaNo ratings yet
- 2 Comparacion BD Phoenix Vitek MicroscanDocument10 pages2 Comparacion BD Phoenix Vitek MicroscanBenjamin MendozaNo ratings yet
- Bio Fertilizer Udyami - Org.inDocument16 pagesBio Fertilizer Udyami - Org.inAndreea DeeaNo ratings yet
- Generation and Validation of Standard Operating Procedure For Dissolution ApparatusDocument18 pagesGeneration and Validation of Standard Operating Procedure For Dissolution ApparatusAbhishek JhaNo ratings yet
- 0707-0712 (1117) Microbiological Best Laboratory PracticesDocument7 pages0707-0712 (1117) Microbiological Best Laboratory PracticesDr usama El ShafeyNo ratings yet
- Antimicrobial Effectiveness Test GMP InvestigationsDocument8 pagesAntimicrobial Effectiveness Test GMP Investigationsgge2502No ratings yet
- Documentation Required For Periodic GMP Compliance Inspection Annex 1 JPDocument5 pagesDocumentation Required For Periodic GMP Compliance Inspection Annex 1 JPspam_discardNo ratings yet
- Validation of MicrobiologicalDocument26 pagesValidation of MicrobiologicalOsman AitaNo ratings yet
- User and Service Manual: Esafe Class Ii Biological Safety CabinetDocument94 pagesUser and Service Manual: Esafe Class Ii Biological Safety Cabinetnihad nasanNo ratings yet
- Portfolio, Program, and Project Management in the Pharmaceutical and Biotechnology IndustriesFrom EverandPortfolio, Program, and Project Management in the Pharmaceutical and Biotechnology IndustriesPete HarpumNo ratings yet
- Microbiological Safety CabinetsDocument14 pagesMicrobiological Safety CabinetsNurul FaizaahNo ratings yet
- 1228 5 PDFDocument5 pages1228 5 PDFdeepanmb007No ratings yet
- Nano-Ipriflavone for OsteoporosisDocument9 pagesNano-Ipriflavone for OsteoporosisChaitrashree GururajNo ratings yet
- MHL-VLP-XX Fumigation ValidationDocument10 pagesMHL-VLP-XX Fumigation ValidationMedicare Hygiene LimitedNo ratings yet
- HPLC Method For Benzalkonium ChlorideDocument12 pagesHPLC Method For Benzalkonium ChlorideFadhlan ArifinNo ratings yet
- E Cosmetics Rules, 2020Document64 pagesE Cosmetics Rules, 2020Aby BabyNo ratings yet
- WP en Calibration Safe Weighing LR 05 2015Document6 pagesWP en Calibration Safe Weighing LR 05 2015Fadhlan ArifinNo ratings yet
- Sampling SurfaceDocument29 pagesSampling SurfaceFadhlan ArifinNo ratings yet
- GC Method For BKCDocument4 pagesGC Method For BKCFadhlan ArifinNo ratings yet
- EN - 12469 (2000) - (E) BSC RequirmentDocument8 pagesEN - 12469 (2000) - (E) BSC RequirmentFadhlan ArifinNo ratings yet
- Module One - Micro and Contamination Control - Rev02Document58 pagesModule One - Micro and Contamination Control - Rev02Fadhlan ArifinNo ratings yet
- Quantofix Test Strips - OverviewDocument2 pagesQuantofix Test Strips - OverviewFadhlan ArifinNo ratings yet
- Xylitol Sebagai AntibakteriDocument7 pagesXylitol Sebagai AntibakteriFadhlan ArifinNo ratings yet
- Applied and Environmental Microbiology-1968-Bühlmann-1919.fullDocument5 pagesApplied and Environmental Microbiology-1968-Bühlmann-1919.fullFadhlan ArifinNo ratings yet
- MF MethodDocument49 pagesMF MethodFadhlan ArifinNo ratings yet
- Micro QC & Hygiene Risk - FINALDocument49 pagesMicro QC & Hygiene Risk - FINALFadhlan ArifinNo ratings yet
- PZ Cussons - Industrial Hygiene Training Module Four Cleaning and SanitizationDocument32 pagesPZ Cussons - Industrial Hygiene Training Module Four Cleaning and SanitizationFadhlan ArifinNo ratings yet
- Water Systems for Personal Care Products; How to Meet Microbiological Quality RequirementsDocument66 pagesWater Systems for Personal Care Products; How to Meet Microbiological Quality RequirementsFadhlan ArifinNo ratings yet
- Antimicrobial Effects of Sodium Benzoate, Sodium Nitrite and Potassium Sorbate and Their Synergistic Action in VitroDocument6 pagesAntimicrobial Effects of Sodium Benzoate, Sodium Nitrite and Potassium Sorbate and Their Synergistic Action in VitroFadhlan ArifinNo ratings yet
- BAM: Staphylococcus Aureus: Bacteriological Analytical ManualDocument5 pagesBAM: Staphylococcus Aureus: Bacteriological Analytical ManualFadhlan ArifinNo ratings yet
- Microbank™-Dry: Product Code Pl.172Document2 pagesMicrobank™-Dry: Product Code Pl.172Fadhlan ArifinNo ratings yet
- FSD 17932Document15 pagesFSD 17932Fadhlan ArifinNo ratings yet
- 32373727Document89 pages32373727Fadhlan ArifinNo ratings yet
- 2080 PDFDocument2 pages2080 PDFFadhlan ArifinNo ratings yet
- Previews 2013360 PreDocument13 pagesPreviews 2013360 PreEko Setyo BudiNo ratings yet
- APA Style Guide for Student PapersDocument24 pagesAPA Style Guide for Student PapersHeureuse MamanNo ratings yet
- Choosing the Right Career and Job RequirementsDocument2 pagesChoosing the Right Career and Job RequirementsdinnahNo ratings yet
- Application Format For Child CustodyDocument2 pagesApplication Format For Child CustodyDHUP CHAND JAISWAL100% (3)
- Fault Tracing: FMI 3: Checking The Sensor CircuitDocument1 pageFault Tracing: FMI 3: Checking The Sensor Circuituser1No ratings yet
- Slide BP Texas City RefineryDocument20 pagesSlide BP Texas City Refineryamaleena_muniraNo ratings yet
- Manual de Reparacion TXV75Document16 pagesManual de Reparacion TXV75Ovh MaquinariasNo ratings yet
- Physical ExaminationDocument7 pagesPhysical ExaminationCha CulveraNo ratings yet
- By Pass System in The Dry ProcessDocument34 pagesBy Pass System in The Dry Processfaheemqc100% (1)
- Acromegaly: Excess Growth Hormone SecretionDocument4 pagesAcromegaly: Excess Growth Hormone SecretionKavita PathakNo ratings yet
- Time and Motion Study of OPDDocument15 pagesTime and Motion Study of OPDsaurabh100% (1)
- IB Student's Soil Systems GuideDocument7 pagesIB Student's Soil Systems GuideYohanes Sumantri RatnodiyantoNo ratings yet
- Biosample Urine Sample Collection Protocol Infant v2Document2 pagesBiosample Urine Sample Collection Protocol Infant v2api-531349549No ratings yet
- Subsea Cable Floats: Rising To Your Undersea ChallengesDocument1 pageSubsea Cable Floats: Rising To Your Undersea ChallengesMAURICIO DE LOS SANTOS HERNANDEZNo ratings yet
- Nigelaycardo 1Document8 pagesNigelaycardo 1ANGELICA AYCARDO FLORESNo ratings yet
- Medical Certificate: (Coaches, Assistant Coaches, Chaperone)Document1 pageMedical Certificate: (Coaches, Assistant Coaches, Chaperone)Keith Marinas Serquina100% (1)
- Differential Pressure TransmitterDocument14 pagesDifferential Pressure TransmitterZainab KadhemNo ratings yet
- Lab Report 2Document5 pagesLab Report 2adrianeNo ratings yet
- There Came A Gypsy RidingDocument45 pagesThere Came A Gypsy RidingMartin McNelisNo ratings yet
- PN15CS1S01Document4 pagesPN15CS1S01joadNo ratings yet
- Umer Afzal 70073638 Ass# 1Document3 pagesUmer Afzal 70073638 Ass# 1Muhammad Umer AfzalNo ratings yet
- The Morning Calm Korea Weekly - Oct. 14, 2005Document26 pagesThe Morning Calm Korea Weekly - Oct. 14, 2005Morning Calm Weekly NewspaperNo ratings yet
- Why encouraging entrepreneurship to boost economies is flawedDocument9 pagesWhy encouraging entrepreneurship to boost economies is flawedLaureanoNo ratings yet
- SG Salary Guide 2021-22Document66 pagesSG Salary Guide 2021-22Gilbert ChiaNo ratings yet
- Inverter Controlled Mig Welding-Machine YD-400GT3 Operating ManualDocument53 pagesInverter Controlled Mig Welding-Machine YD-400GT3 Operating ManualsunhuynhNo ratings yet
- 7Document40 pages7Felipe RichardiosNo ratings yet
- MODULE 9: Personal Relationships: Albert Abarabar, Erica Soriano, Lloyd Sarandi, Julius Dela CruzDocument18 pagesMODULE 9: Personal Relationships: Albert Abarabar, Erica Soriano, Lloyd Sarandi, Julius Dela CruzMark Gil GuillermoNo ratings yet
- Pro Boxberg en DownloadDocument6 pagesPro Boxberg en Downloadftzo3439No ratings yet
- Vegetarianism A Salvation Issue?Document7 pagesVegetarianism A Salvation Issue?ChetiweKapilaNo ratings yet
- Glycerol PDFDocument3 pagesGlycerol PDFTushar GaikarNo ratings yet