Professional Documents
Culture Documents
Grade 10 Chemistry Week 3 Lesson 1 Worksheet 1 and Solutions
Uploaded by
Nikoli MajorOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Grade 10 Chemistry Week 3 Lesson 1 Worksheet 1 and Solutions
Uploaded by
Nikoli MajorCopyright:
Available Formats
Ministry of Education
Secondary Engagement Program
Grade 10
Chemistry
Week 3 Lesson 1 - Worksheet
Use the periodic table and your knowledge of periodic trends to answer the following questions.
1. Which atom in each pair has the larger atomic radius?
a) Li or K b) Ca or Ni c) Ga or B d) O or C e) Cl or Br
f) Be or Ba g) Si or S h) Fe or Au
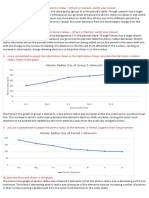
2. What is the periodic trend for atomic size from top to bottom in a group?
3. Which element in each pair has a larger ionization energy?
a) Na or O b) Be or Ba c) Ar or F d) Cu or Ra e) I or Ne
f) K or V g) Ca or Fr h) W or Se
4. What is the periodic trend for first ionization energy?
5. What is ionization energy? What is first ionization energy?
6. Arrange the following groups of elements in order of increasing ionization energy.
a) Be, Mg, Sr b) Bi, Cs, Ba c) Na, Al, S
7. Which element in each pair has a higher electronegativity value?
a) Cl, F b) C, N c) Mg, Ne d) As, Ca
8. What is the periodic trend for electronegativity?
9. Why do elements in the same family generally have similar properties?
10. Why does the atomic radius increase as you go down a group or family?
Ministry of Education
Secondary Engagement Program
Grade 10
Chemistry
Week 3 Lesson 1: Worksheet - Answers
1. Which atom in each pair has the larger atomic radius?
a) Li or K b) Ca or Ni c) Ga or B d) O or C e) Cl or Br
f) Be or Ba g) Si or S h) Fe or Au
2. What is the periodic trend for atomic size from top to bottom in a group?
Top to bottom: increases due to adding energy levels
3. Which element in each pair has a larger ionization energy?
a) Na or O b) Be or Ba c) Ar or F d) Cu or Ra e) I or Ne
f) K or V g) Ca or Fr h) W or Se
4. What is the periodic trend for first ionization energy?
It increases going from left to right due to increased nuclear charge, and decreases going
down a group due to increased distance from the nucleus and increased shielding.
5. What is ionization energy? What is first ionization energy?
The amount of energy needed to remove an electron from an atom. First ionization
energy is the energy required to remove a single valence electron from an atom.
6. Arrange the following groups of elements in order of increasing ionization energy.
a) Be, Mg, Sr b) Bi, Cs, Ba c) Na, Al, S
high, middle, low high, low, middle low, middle, high
7. Which element in each pair has a higher electronegativity value?
a) Cl, F b) C, N c) Mg, Ne d) As, Ca
8. What is the periodic trend for electronegativity? Increases going left to right, decreases
going down.
9. Why do elements in the same family generally have similar properties?
Because they have similar electron configurations and the same number of valence
electrons. Because valence electrons are responsible for most of the chemistry we
observe, this similarity causes the properties of the elements to also be similar.
You might also like
- Periodic Trends Worksheet 1Document1 pagePeriodic Trends Worksheet 1Soham ShindeNo ratings yet
- Periodic Trends Worksheet 1 AnswersDocument1 pagePeriodic Trends Worksheet 1 AnswersChristine Angeline C. Cabasan75% (4)
- Periodic Trends WorksheetDocument2 pagesPeriodic Trends WorksheetHannah LeeNo ratings yet
- Periodic Trends WorksheetDocument2 pagesPeriodic Trends Worksheetabdul_imran_17No ratings yet
- Periodic Trends Worksheet: Directions: Use Your Notes To Answer The Following QuestionsDocument2 pagesPeriodic Trends Worksheet: Directions: Use Your Notes To Answer The Following QuestionsAizelle TarataraNo ratings yet
- Periodic Table and Periodicity of PropertiesDocument5 pagesPeriodic Table and Periodicity of PropertiesSaad shamimNo ratings yet
- Last Moment PreparationRevision Part IIDocument142 pagesLast Moment PreparationRevision Part IIsufyiansafdarNo ratings yet
- Periodicity Review AnswersDocument2 pagesPeriodicity Review Answersapi-263909505100% (1)
- Atomic Radius and Electronegativity - QuestionsDocument4 pagesAtomic Radius and Electronegativity - QuestionsAnonymous Mj0pfScNo ratings yet
- 5 Trends in The Periodic TableDocument4 pages5 Trends in The Periodic TableCris CorsinoNo ratings yet
- Atomic, Ionic Size, I.E Worksheet PDFDocument2 pagesAtomic, Ionic Size, I.E Worksheet PDFmaitha91No ratings yet
- Chemistry Semester 1 Final Study Guide KeyDocument7 pagesChemistry Semester 1 Final Study Guide Keyalexanderhdinh50% (2)
- p2 Chemistry PDFDocument4 pagesp2 Chemistry PDFSAP BWNo ratings yet
- Periodic Trends Practice Test KEYDocument3 pagesPeriodic Trends Practice Test KEYKateAshleyLiaoNo ratings yet
- Worksheet 1: Periodic Properties and Variation of PropertiesDocument3 pagesWorksheet 1: Periodic Properties and Variation of Propertiessai hitheshNo ratings yet
- Periodic Trends Graphing ActivityDocument6 pagesPeriodic Trends Graphing ActivityKuro NekoNo ratings yet
- Ionisation EnergyDocument36 pagesIonisation Energykanaiya2006No ratings yet
- Periodic Table - Periodic Properties & Variations of PropertiesDocument34 pagesPeriodic Table - Periodic Properties & Variations of PropertieskumarvaradarajanNo ratings yet
- Sci 9 M5Document27 pagesSci 9 M5Shen shenNo ratings yet
- Periodic TrendsDocument2 pagesPeriodic TrendsKaoutar MazouzNo ratings yet
- Science 9 Quiz# 1 2nd QDocument3 pagesScience 9 Quiz# 1 2nd QEmmanuel LlonaNo ratings yet
- Chapter 5 Periodic Classification of ElementsDocument9 pagesChapter 5 Periodic Classification of ElementsasuhassNo ratings yet
- Chapter 1 Periodic PropertiesDocument34 pagesChapter 1 Periodic PropertiesMayank Mourya100% (1)
- Periodic Table PNOC Reviewer 30 QuestionsDocument2 pagesPeriodic Table PNOC Reviewer 30 QuestionsMark Jay AquilloNo ratings yet
- Chemistry 7th Edition McMurry Solutions Manual DownloadDocument6 pagesChemistry 7th Edition McMurry Solutions Manual DownloadRoger Wright100% (21)
- Answer Key XI CH 3 Worksheet 2Document5 pagesAnswer Key XI CH 3 Worksheet 2iroonmaan123No ratings yet
- Periodicity Chemistry Worksheet: A. Periodic TableDocument9 pagesPeriodicity Chemistry Worksheet: A. Periodic TableRhea FrancisNo ratings yet
- Periodic Table Trend QuizDocument2 pagesPeriodic Table Trend Quiz안동현No ratings yet
- 10 Science NcertSolutions Chapter 5 ExercisesDocument4 pages10 Science NcertSolutions Chapter 5 ExercisesContacts nilNo ratings yet
- STPM Chemistry Form 6 NotesDocument5 pagesSTPM Chemistry Form 6 NotesAfz Min100% (3)
- Cfe Higher Chemistry - PeriodicityDocument34 pagesCfe Higher Chemistry - Periodicityiapm0708No ratings yet
- Summative Assessment Structure and Properties of MatterDocument4 pagesSummative Assessment Structure and Properties of MatterKayla RhodesNo ratings yet
- Dalton's Law of Partial PressureDocument4 pagesDalton's Law of Partial PressureAgyao Yam FaithNo ratings yet
- ch01 ChemDocument8 pagesch01 Chemtraderakash32No ratings yet
- SME Chemistry Topic 3 NotesDocument36 pagesSME Chemistry Topic 3 NotesAlyasin FrougaNo ratings yet
- Chapter 3 Practice QuestionsDocument6 pagesChapter 3 Practice QuestionsRanem Ahmed Nasser Al ShibaniNo ratings yet
- In Class Practice On Periodic Trends WsDocument3 pagesIn Class Practice On Periodic Trends WsFern HofileñaNo ratings yet
- PT - Question 2Document6 pagesPT - Question 2Mila FactorNo ratings yet
- Periodic Trends C12 2 07Document13 pagesPeriodic Trends C12 2 07Kuro NekoNo ratings yet
- Periodic TrendsDocument3 pagesPeriodic TrendsJessica ShinNo ratings yet
- Chemistry - Textbook Answers Chapter 5Document20 pagesChemistry - Textbook Answers Chapter 5angelina_boseNo ratings yet
- Conceptual Chemistry 5Th Edition Suchocki Test Bank Full Chapter PDFDocument36 pagesConceptual Chemistry 5Th Edition Suchocki Test Bank Full Chapter PDFsuzanne.guillory241100% (9)
- CBSE Class 10 Science Chapter 5 NCERT Solutions 2022 - Free PDFDocument7 pagesCBSE Class 10 Science Chapter 5 NCERT Solutions 2022 - Free PDFMuzafar ahmadNo ratings yet
- Chem11 SM 1 7Document2 pagesChem11 SM 1 7dudoocandrawNo ratings yet
- Periodic Properties WorksheetDocument1 pagePeriodic Properties WorksheetMohd ArsalanNo ratings yet
- Chapter 5 Periodic Trends - KeyDocument10 pagesChapter 5 Periodic Trends - KeyAref DahabrahNo ratings yet
- 20 Periodic Table Trends KEYDocument3 pages20 Periodic Table Trends KEYTheresa Rebullos BileNo ratings yet
- Screenshot 2023-11-04 at 4.12.18 PMDocument45 pagesScreenshot 2023-11-04 at 4.12.18 PMmalakbasahalNo ratings yet
- Atomic Structure Bohr Model Periodic Trends Student Notes Practice Problems 1Document10 pagesAtomic Structure Bohr Model Periodic Trends Student Notes Practice Problems 1api-255434272No ratings yet
- CHM111E 1.1.1 - Periodic Table and Its Trends PDFDocument43 pagesCHM111E 1.1.1 - Periodic Table and Its Trends PDFanton petrovNo ratings yet
- Civil Engineering DefinitionsDocument1 pageCivil Engineering DefinitionsJustinorin94No ratings yet
- Periodic Table Trends Worksheet - AnswersDocument3 pagesPeriodic Table Trends Worksheet - AnswersNihalAbou-Ghaly100% (1)
- Lesson PlanDocument4 pagesLesson PlanElgaliza Karina DeviNo ratings yet
- Science - Test Paper - X - CH 5Document4 pagesScience - Test Paper - X - CH 5Víshál RánáNo ratings yet
- Ionic Bonding ReviewDocument54 pagesIonic Bonding ReviewAxle JavierNo ratings yet
- Analytical Chemistry-2: Action of Alkali On Certain MetalsDocument3 pagesAnalytical Chemistry-2: Action of Alkali On Certain MetalsManju YadavNo ratings yet
- Exercise Soln of Periodic ElementsDocument13 pagesExercise Soln of Periodic ElementsiTutor Classes BapiNo ratings yet
- Practice Makes Perfect in Chemistry: Chemical Bonding with AnswersFrom EverandPractice Makes Perfect in Chemistry: Chemical Bonding with AnswersRating: 5 out of 5 stars5/5 (1)
- Practice Makes Perfect in Chemistry: Chemical BondingFrom EverandPractice Makes Perfect in Chemistry: Chemical BondingRating: 5 out of 5 stars5/5 (3)
- Grade 10 Chemistry Week 1 Worksheet 1 and SolutionsDocument2 pagesGrade 10 Chemistry Week 1 Worksheet 1 and SolutionsNikoli MajorNo ratings yet
- Grade 10 Chemistry Week 1Document3 pagesGrade 10 Chemistry Week 1Nikoli MajorNo ratings yet
- AGR 3204 Worksheet III 2023Document4 pagesAGR 3204 Worksheet III 2023Nikoli MajorNo ratings yet
- Grade 10 Chemistry Week 3 Lesson 2 Worksheet 2 and SolutionsDocument7 pagesGrade 10 Chemistry Week 3 Lesson 2 Worksheet 2 and SolutionsNikoli MajorNo ratings yet
- Grade 10 Chemistry Week 4 Lesson 1Document3 pagesGrade 10 Chemistry Week 4 Lesson 1Nikoli MajorNo ratings yet
- Grade 10 Chemistry Week 3 Lesson 1Document4 pagesGrade 10 Chemistry Week 3 Lesson 1Nikoli MajorNo ratings yet
- Grade 10 Chemistry Week 1 Lesson 2 Worksheet 1 and SlutionsDocument2 pagesGrade 10 Chemistry Week 1 Lesson 2 Worksheet 1 and SlutionsNikoli MajorNo ratings yet
- Grade 10 Chemistry Week 7 Lesson 1Document2 pagesGrade 10 Chemistry Week 7 Lesson 1Nikoli MajorNo ratings yet
- Grade 10 Chemistry Week 4 Lesson 1 Worksheet 1 and SolutionsDocument5 pagesGrade 10 Chemistry Week 4 Lesson 1 Worksheet 1 and SolutionsNikoli MajorNo ratings yet
- Grade 10 Chemistry Week 8 Lesson 1Document5 pagesGrade 10 Chemistry Week 8 Lesson 1Nikoli MajorNo ratings yet
- Grade 10 Chemistry Week 7 Lesson 1 Worksheet 1 and SolutionsDocument5 pagesGrade 10 Chemistry Week 7 Lesson 1 Worksheet 1 and SolutionsNikoli MajorNo ratings yet
- Grade 10 Chemistry Week 8 Lesson 2Document4 pagesGrade 10 Chemistry Week 8 Lesson 2Nikoli MajorNo ratings yet
- Grade 10 Chemistry Week 3 Lesson 2Document2 pagesGrade 10 Chemistry Week 3 Lesson 2Nikoli MajorNo ratings yet
- Grade 10 Chemistry Week 10 Lesson 2 Worksheet 1 and Solutions PDFDocument7 pagesGrade 10 Chemistry Week 10 Lesson 2 Worksheet 1 and Solutions PDFNikoli MajorNo ratings yet
- Grade 10 Chemistry Week 9 Lesson 2 Worksheet 1 and SolutionsDocument2 pagesGrade 10 Chemistry Week 9 Lesson 2 Worksheet 1 and SolutionsNikoli MajorNo ratings yet
- Grade 10 Chemistry Week 9 Lesson 2Document4 pagesGrade 10 Chemistry Week 9 Lesson 2Nikoli MajorNo ratings yet
- 1 BDocument4 pages1 BNikoli MajorNo ratings yet
- Grade 10 Chemistry Week 2 Lesson 2 Worksheet 1 and SolutionsDocument3 pagesGrade 10 Chemistry Week 2 Lesson 2 Worksheet 1 and SolutionsNikoli MajorNo ratings yet
- Grade 10 Chemistry Week 10 Lesson 2Document2 pagesGrade 10 Chemistry Week 10 Lesson 2Nikoli MajorNo ratings yet
- 1 ADocument3 pages1 ANikoli MajorNo ratings yet
- CHM1207 Lab 3 2023 - DRAKES, Tameica (1042436) PDFDocument6 pagesCHM1207 Lab 3 2023 - DRAKES, Tameica (1042436) PDFNikoli MajorNo ratings yet
- Grade 10 Chemistry Week 8 Lesson 1 Worksheet 1 and SolutionsDocument3 pagesGrade 10 Chemistry Week 8 Lesson 1 Worksheet 1 and SolutionsNikoli MajorNo ratings yet
- Lecture 3Document61 pagesLecture 3Nikoli MajorNo ratings yet
- Ministry of Education Secondary Engagement Programme Grade 11 ChemistryDocument4 pagesMinistry of Education Secondary Engagement Programme Grade 11 ChemistryNikoli MajorNo ratings yet
- Lecture 5Document58 pagesLecture 5Nikoli MajorNo ratings yet
- Lecture 6Document79 pagesLecture 6Nikoli MajorNo ratings yet
- Grade 10 Chemistry Week 11 Lesson 1Document7 pagesGrade 10 Chemistry Week 11 Lesson 1Nikoli MajorNo ratings yet
- Grade 10 Chemistry Week 10 Lesson 1Document3 pagesGrade 10 Chemistry Week 10 Lesson 1Nikoli MajorNo ratings yet
- Lecture 4Document116 pagesLecture 4Nikoli MajorNo ratings yet
- Lecture 2Document75 pagesLecture 2Nikoli MajorNo ratings yet
- 2 Visible Spectroscopy - GoodDocument7 pages2 Visible Spectroscopy - GoodOmSilence2651No ratings yet
- Nuclear and Particle Physics: Vocabulary ReviewDocument8 pagesNuclear and Particle Physics: Vocabulary ReviewthamerNo ratings yet
- Chapter 2 RRLDocument5 pagesChapter 2 RRLreynan_12No ratings yet
- Question Bank On Electronic Conf.Document6 pagesQuestion Bank On Electronic Conf.Harsh TyagiNo ratings yet
- Basic Inorganic Chemistry Part 1 Transition Metals - Theories, PropertiesDocument71 pagesBasic Inorganic Chemistry Part 1 Transition Metals - Theories, Propertiesyashaswini tiwariNo ratings yet
- InVia Raman Microscope - Excitation Wavelength OptionsDocument4 pagesInVia Raman Microscope - Excitation Wavelength OptionsSindhuraj MukherjeeNo ratings yet
- Review - 2018 - Final ExamDocument3 pagesReview - 2018 - Final ExamQuang LinhNo ratings yet
- Unit 4 Structure Unfolding TechniquesDocument29 pagesUnit 4 Structure Unfolding TechniquesvijayNo ratings yet
- General Chemistry 1 Quarter 2 Weeks 2 and 3Document17 pagesGeneral Chemistry 1 Quarter 2 Weeks 2 and 3Shalou Beth FlorendoNo ratings yet
- Trick HybridisationDocument26 pagesTrick HybridisationQaisar RiazNo ratings yet
- Study Notes Atomic Structure ModelsDocument5 pagesStudy Notes Atomic Structure ModelsG21 Odtojan, Jhade Ann E.No ratings yet
- Chemistry Malaysian Matriculation Full Notes Amp Slides For Semester 1 and 2 PDFDocument1,743 pagesChemistry Malaysian Matriculation Full Notes Amp Slides For Semester 1 and 2 PDFHaarini100% (1)
- Franck-Hertz ExperimentDocument4 pagesFranck-Hertz ExperimentProfessor VoltNo ratings yet
- Chapter 8 PDFDocument4 pagesChapter 8 PDFChristeena HenryNo ratings yet
- Structure of AtomDocument9 pagesStructure of AtomSharma RichaNo ratings yet
- Keldysh PDFDocument8 pagesKeldysh PDFElektronika PMFNo ratings yet
- 13c-n M-R. Studies of Monomeric Composition and SequenceDocument13 pages13c-n M-R. Studies of Monomeric Composition and SequenceJulio Andrés Arce HerreraNo ratings yet
- Atomic Structure Neet Previous Year Solved Paper.Document8 pagesAtomic Structure Neet Previous Year Solved Paper.MUHAMMAD USAMA MULLA.No ratings yet
- Science Form 4 Chapter 4Document10 pagesScience Form 4 Chapter 4NETAJI RAONo ratings yet
- Nuclear EquationsDocument21 pagesNuclear EquationsAbigail AmorNo ratings yet
- TheoryDocument29 pagesTheoryRajesh JainNo ratings yet
- Covalent Bonding WebquestDocument4 pagesCovalent Bonding Webquestapi-3031203990% (1)
- Introduction To Optical Emission Spectroscopy: Edmund HalászDocument30 pagesIntroduction To Optical Emission Spectroscopy: Edmund HalászKleverton JunioNo ratings yet
- Concept MapDocument3 pagesConcept MapAlyssa BihagNo ratings yet
- Problems and Solutions: Physical ChemistryDocument91 pagesProblems and Solutions: Physical ChemistryChicken ChickenNo ratings yet
- Ass 1 SDFGHJK DFGHJK DFGHJK Ertyuiop SDFGHJKLDocument10 pagesAss 1 SDFGHJK DFGHJK DFGHJK Ertyuiop SDFGHJKLriniz92No ratings yet
- Introduction To Infrared Spectroscopy: D. Jim Livingston Faculty of Chemistry St. John'S CollegeDocument22 pagesIntroduction To Infrared Spectroscopy: D. Jim Livingston Faculty of Chemistry St. John'S CollegeJim LivingstonNo ratings yet
- Interactive Textbook 1 PDF 4 1Document8 pagesInteractive Textbook 1 PDF 4 1api-240094705100% (2)
- Atomic Structure ChemistryDocument143 pagesAtomic Structure ChemistryYoshitha Kuntumalla100% (1)
- Chapter 2 Chemistry For Engineers Final Module 2Document26 pagesChapter 2 Chemistry For Engineers Final Module 2Alex Jr. Rosadiño C.No ratings yet