Professional Documents
Culture Documents
bài tập rate
Uploaded by
Mys Genie100%(1)100% found this document useful (1 vote)
22 views2 pagesCopyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
100%(1)100% found this document useful (1 vote)
22 views2 pagesbài tập rate
Uploaded by
Mys GenieCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
Rate of reaction Exercise What is the rate equation that describes the rate’s
dependence on the concentrations of NO and Cl 2? What is
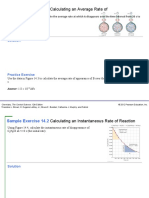
1. A study of the rate of dimerization of C 4H6 gave the the rate constant? What are the orders with respect to each
data shown in the table: reactant?
2C4H6⟶C8H12 5. Hydrogen reacts with nitrogen monoxide to form
Time 0 1600 3200 4800 6200 dinitrogen monoxide (laughing gas) according to the
(s) equation:
[C4H6] 1.00 × 5.04 × 3.37 × 2.53 × 2.08 ×
(M) 10−2 10−3 10−3 10−3 10−3 H2(g)+2NO(g)⟶N2O(g)+H2O(g)H2(g)
a. Determine the average dimerization rate between 0 +2NO(g)⟶N2O(g)+H2O(g)
s and 1600 s, and between 1600 s and 3200 s.
Determine the rate equation, the rate constant, and the
b. Estimate the instantaneous dimerization rate at
orders with respect to each reactant from the following data:
3200 s from a time graph versus [C 4H6]. What are the units
of this rate?
[NO] (M) 0.30 0.60 0.60
2. How much and in what direction will each of the [H2] (M) 0.35 0.35 0.70
following affect the rate of the reaction: CO(g) Rate 2.835 × 1.134 × 2.268 ×
+NO2(g)⟶CO2(g)+NO(g)CO(g)+NO2(g)⟶CO2(g) (mol/L/s) 10−3 10−2 10−2
+NO(g) if the rate law for the reaction
is rate=k[NO2]2rate=k[NO2]2? 6. For the reaction A⟶B+CA⟶B+C, the following
data were obtained at 30 °C:
a. Decreasing the pressure of NO2 from 0.50 atm to
0.250 atm. [A] (M) 0.230 0.356 0.557
b. Increasing the concentration of CO from 0.01 M to Rate (mol/L/s) 4.17 × 10−4 9.99 × 10−4 2.44 × 10−3
0.03 M. a. What is the order of the reaction with respect to [A],
and what is the rate equation?
b. What is the rate constant?
3. Nitrosyl chloride, NOCl, decomposes to NO and
Cl2. 7. The following data have been determined for the
reaction:
2NOCl(g)⟶2NO(g)+Cl2(g)2NOCl(g)⟶2NO(g)+Cl2(g) I−+OCl−⟶IO−+Cl−I−+OCl−⟶IO−+Cl−
Determine the rate equation, the rate constant, and the 1 2 3
overall order for this reaction from the following data: [I−]initial[I−]initial (M) 0.10 0.20 0.30
[OCl−]initial[OCl−]initial (M 0.050 0.05 0.010
[NOCl] (M) 0.10 0.20 0.30 ) 0
Rate 8.0 × 10−10 3.2 × 10−9 7.2 × 10−9 Rate (mol/L/s) 3.05 × 6.20 1.83
(mol/L/h) 10−4 × ×
10−4 10−4
Nitrogen(II) oxide reacts with chlorine according to the
equation: Determine the rate equation and the rate constant for this
reaction.
8. In the reaction
2NO(g)+Cl2(g)⟶2NOCl(g)2NO(g)+Cl2(g)⟶2NOCl(g)
2NO+Cl2→2NOCl2NO+Cl2→2NOCl
4. The following initial rates of reaction have been
observed for certain reactant concentrations:
the reactants and products are gases at the temperature of
the reaction. The following rate data were measured for
[NO] (mol/L1) [Cl2] (mol/L) Rate (mol/L/h)
three experiments:
0.50 0.50 1.14
1.00 0.50 4.56
Initial p{NO} Initial p{Cl2} Initial rate
1.00 1.00 9.12
(atm) (atm) (moles of A consumed
atm sec-1)
0.50 0.50 5.1 x 10-3
1.0 1.0 4.0 x 10-2
0.50 1.0 1.0 x 10-2
a. From these data, write the rate equation for this gas
reaction. What order is the reaction in NO, Cl 2, and overall?
b. Calculate the specific rate constant for this reaction.
9. The activation energy of an
elementary chemical reaction is 40 kcal
mol-1. Determine:
i. The increase in the reaction rate when the
temperature increases from 300ºC· to 350ºC
ii. The increase in temperature necessary to double
the rate of the reaction at 300ºC
10. A reaction A+B+C → Products yielded the
following data:
Initial concentration (mmol L
Experiment [A]0 [B]0 [C]0
1 1.25 1.25 1.25
2 2.50 1.25 1.25
3 1.25 3.02 1.25
4 1.25 3.02 3.75
i. Write the rate law for this reaction
ii. If the initial reactant concentrations were [A] 0 =
3.01 mmol L-1, [B]0 = 1.00 mmol L-1 and [C]0
=1.15 mmol L-1, indicate what would be the initial rate of
disappearance of A.
11. The following table shows how the concentration
of HI varies in the reaction:
2 HI(g) → H2(g) +I2(g).
Time (s) 0 1000 2000
[HI] (mol/L) 1.00 0.112 0.061
Determine:
i. The global reaction order.
ii. The kinetic constant for the rate of disappearance
of HI.
iii. The kinetic constant for the rate law
(independent of the reactant).
You might also like
- CH13 Practice ExamDocument8 pagesCH13 Practice ExamAnonymous WI0nbsNo ratings yet
- Fractionation SystemsDocument8 pagesFractionation SystemsKha Damayantirika Tsf 'reall'No ratings yet
- DRRR - Module 9Document26 pagesDRRR - Module 9Kennedy Fieldad Vagay67% (6)
- PLTL Ch. 16 AssignmentDocument6 pagesPLTL Ch. 16 AssignmentJules BrunoNo ratings yet
- Tutorial 2 StudentDocument6 pagesTutorial 2 StudentIrsyad KamilNo ratings yet
- Petroleum and Gas ProcessingDocument830 pagesPetroleum and Gas ProcessingVeronica Strotsen100% (4)
- 08a. Chemical Kinetics SheetDocument33 pages08a. Chemical Kinetics SheetVIKRANTH KUMAR JAKKOJUNo ratings yet
- 102 MSJC 13Document11 pages102 MSJC 13noelNo ratings yet
- PENEX PROCESS TECHNOLOGY OVERVIEWDocument34 pagesPENEX PROCESS TECHNOLOGY OVERVIEWSALAM ALINo ratings yet
- Tutorial 2Document2 pagesTutorial 2EreenNo ratings yet
- Chemistry Worksheet 2Document3 pagesChemistry Worksheet 2LemontNo ratings yet
- Chm271 - Tutorial 5 - Chemical KineticsDocument6 pagesChm271 - Tutorial 5 - Chemical Kineticsfiefy zmrNo ratings yet
- Exercises Unit4 1Document3 pagesExercises Unit4 1Mabe ArcentalesNo ratings yet
- Chapter 1 Reaction KineticsDocument8 pagesChapter 1 Reaction KineticsDinesh RamaNo ratings yet
- Assignment 3 Chemical KineticsDocument1 pageAssignment 3 Chemical Kineticsvegamaharajfaith02No ratings yet
- Chemcal Kinetics (Tutorial Questions)Document3 pagesChemcal Kinetics (Tutorial Questions)renNo ratings yet
- Chemistry File 4Document4 pagesChemistry File 4Pawan Kumar100% (1)
- Rate Laws and Reaction Kinetics TutorialDocument2 pagesRate Laws and Reaction Kinetics TutorialKamilia AfiqahNo ratings yet
- MCD4390 Week 10 Tutorial QuestionsDocument5 pagesMCD4390 Week 10 Tutorial QuestionsGabbar100% (1)
- Kinetics practice problemsDocument7 pagesKinetics practice problemsJomari GaliasNo ratings yet
- Topic 16 Past Paper Questions 2011Document15 pagesTopic 16 Past Paper Questions 2011nadia sykesNo ratings yet
- EDUC 3136 A TeST 1 Reaction Kinetics 2023 PDFDocument11 pagesEDUC 3136 A TeST 1 Reaction Kinetics 2023 PDFKgaugelo FenyaneNo ratings yet
- Tutorial 4Document3 pagesTutorial 4aliesyaNo ratings yet
- DSCVDSCVDocument15 pagesDSCVDSCVnehelet920No ratings yet
- Rates of Reaction Suroviec Spring 2014Document43 pagesRates of Reaction Suroviec Spring 2014enesffsNo ratings yet
- Tutorial-Manual CH1002Document18 pagesTutorial-Manual CH1002Gift Chulu100% (2)
- Chap 12-13Document5 pagesChap 12-13noviNo ratings yet
- Ap ChemDocument3 pagesAp ChemEthan NguyenNo ratings yet
- 163Ch11 13Document7 pages163Ch11 13Aaron BautistaNo ratings yet
- Tutorial Sheet 4 - CHEMICAL KINETICSDocument4 pagesTutorial Sheet 4 - CHEMICAL KINETICSSaviour SichizyaNo ratings yet
- Kinetics Worksheet AnswersDocument7 pagesKinetics Worksheet AnswerslinaNo ratings yet
- Kinetics Revision Worksheet 2 (Solutions)Document8 pagesKinetics Revision Worksheet 2 (Solutions)Lee Jun HuiNo ratings yet
- Chapter 4Document3 pagesChapter 4khalidNo ratings yet
- Chem 312 Test 1 2013 MemoDocument11 pagesChem 312 Test 1 2013 Memomatloa71No ratings yet
- ap-chem_kinetics_fr2Document11 pagesap-chem_kinetics_fr2hylee102594No ratings yet
- Determining The Rate Law From Experimental DataDocument45 pagesDetermining The Rate Law From Experimental Datasospeter barasaNo ratings yet
- Kinetics Practice ProblemsDocument4 pagesKinetics Practice ProblemsLuke MeredithNo ratings yet
- K00337 - 20180906121226 - Exercises 1Document3 pagesK00337 - 20180906121226 - Exercises 1andiana siona100% (1)
- Sk0014 Physical & Iinorganic Chemistry Tutorial 4: Reaction KineticsDocument2 pagesSk0014 Physical & Iinorganic Chemistry Tutorial 4: Reaction KineticsNeil8353 GgNo ratings yet
- Reaction Kinetics TutorialDocument7 pagesReaction Kinetics Tutoriallorraine_cuaNo ratings yet
- Chap0-Dong Hoa HocDocument24 pagesChap0-Dong Hoa HocVan Nguyen Phuong NganNo ratings yet
- 2011 Problem Set 7 SolutionsDocument4 pages2011 Problem Set 7 SolutionsSiti AnisNo ratings yet
- Homework 2 (Ch11) - 2020Document4 pagesHomework 2 (Ch11) - 2020Keiko CheungNo ratings yet
- Tutorial (Kinetics) AnswersDocument4 pagesTutorial (Kinetics) Answersoh khang chiangNo ratings yet
- CHE1010 Tutorial Sheet 7Document3 pagesCHE1010 Tutorial Sheet 7Chimuka Onson MapikiNo ratings yet
- 12th Revision Test Chap. 1,2&3Document4 pages12th Revision Test Chap. 1,2&3Bloody DemonNo ratings yet
- 12 - Kinetics 2-7Document2 pages12 - Kinetics 2-7vikyappleNo ratings yet
- Chemical Kinetics: Joshua Micole Felizarta, LPTDocument25 pagesChemical Kinetics: Joshua Micole Felizarta, LPTArvin SayotoNo ratings yet
- Chemical Kinetics Rate EquationsDocument2 pagesChemical Kinetics Rate EquationsMOHAMED HISHAMNo ratings yet
- Chemical Kinetics Board Questions 2010Document5 pagesChemical Kinetics Board Questions 2010amone nNo ratings yet
- Exercises KineticsDocument7 pagesExercises KineticsFahad AlasmiNo ratings yet
- CHEM 1412 homework solutions for reaction ratesDocument7 pagesCHEM 1412 homework solutions for reaction ratesSerpicoNo ratings yet
- Thermo, Kinetics, Electro Review ANSWERSDocument10 pagesThermo, Kinetics, Electro Review ANSWERSDavid LeeNo ratings yet
- CHM 096 Tutorial 1Document4 pagesCHM 096 Tutorial 1Muhammad ShafiqNo ratings yet
- CH 14 Kinetics Part1 WebDocument39 pagesCH 14 Kinetics Part1 Webblue educationNo ratings yet
- CHEM 178 28-40 Miller Ormiller Chem178 FinalExamReviewAnswersDocument4 pagesCHEM 178 28-40 Miller Ormiller Chem178 FinalExamReviewAnswersjassi bNo ratings yet
- Chemical Kinetics Rate Constants and Reaction OrdersDocument3 pagesChemical Kinetics Rate Constants and Reaction OrdersVANESSA JOHNS CLASS XINo ratings yet
- STEM-Dawgs Workshops – Practice Exam 3 KEY CHEM 142ADocument3 pagesSTEM-Dawgs Workshops – Practice Exam 3 KEY CHEM 142AAlana Yudha-WrightNo ratings yet
- Chapter 2 - Chemical KineticsDocument92 pagesChapter 2 - Chemical KineticssabNo ratings yet
- 10 Determining Rate Law Expression From Data S201 1Document4 pages10 Determining Rate Law Expression From Data S201 1AatishImrozNo ratings yet
- Chemical KineticsDocument22 pagesChemical KineticsEleanorNo ratings yet
- Fall 2020 CHEM 112 Exam 3 Practice ProblemsDocument12 pagesFall 2020 CHEM 112 Exam 3 Practice Problemskimber brownNo ratings yet
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNo ratings yet
- ZN CX NynanoparticlearraysderivedfromametDocument8 pagesZN CX NynanoparticlearraysderivedfromametMys GenieNo ratings yet
- Fabrication and Photocatalytic Property of Novel SDocument9 pagesFabrication and Photocatalytic Property of Novel SMys GenieNo ratings yet
- Synthesis of A Highly E Cient Biocl Single-Crystal Nanodisk Photocatalyst With Exposing (001) FacetsDocument7 pagesSynthesis of A Highly E Cient Biocl Single-Crystal Nanodisk Photocatalyst With Exposing (001) FacetsMys GenieNo ratings yet
- 2009 JPCPB CHLDocument8 pages2009 JPCPB CHLMys GenieNo ratings yet
- Tuning Band Gaps and Optical Absorption of Biocl Through Doping and Strain Insight Form DFT CalculationsDocument7 pagesTuning Band Gaps and Optical Absorption of Biocl Through Doping and Strain Insight Form DFT CalculationsMys GenieNo ratings yet
- Twin-Graphene As A Promising Anode Material For Na-Ion Rechargeable BatteriesDocument8 pagesTwin-Graphene As A Promising Anode Material For Na-Ion Rechargeable BatteriesMys GenieNo ratings yet
- ZNS H2S Photocat H2Document8 pagesZNS H2S Photocat H2Mys GenieNo ratings yet
- Accepted Article: Title: Bi5+, Bi (3-X) +, and Oxygen Vacancy Induced Bioclxi1-X SolidDocument14 pagesAccepted Article: Title: Bi5+, Bi (3-X) +, and Oxygen Vacancy Induced Bioclxi1-X SolidMys GenieNo ratings yet
- 1 s2.0 S004565352103592X MainDocument37 pages1 s2.0 S004565352103592X MainMys GenieNo ratings yet
- Biologically Synthesized BlackDocument21 pagesBiologically Synthesized BlackMys GenieNo ratings yet
- Facile Fabrication of BiOIBiOCl Immobilized FilmsDocument11 pagesFacile Fabrication of BiOIBiOCl Immobilized FilmsMys GenieNo ratings yet
- bài tập rate 2Document12 pagesbài tập rate 2Mys GenieNo ratings yet
- 1 s2.0 S1383586622018913 MainDocument16 pages1 s2.0 S1383586622018913 MainMys GenieNo ratings yet
- 1 s2.0 S2213343720308320 MainDocument12 pages1 s2.0 S2213343720308320 MainMys GenieNo ratings yet
- Ceri D 23 03980Document50 pagesCeri D 23 03980Mys GenieNo ratings yet
- Coupling Photocatalytic Fuel Cell Based On S-Scheme G-C N /tnasDocument10 pagesCoupling Photocatalytic Fuel Cell Based On S-Scheme G-C N /tnasMys GenieNo ratings yet
- CarbonLetter B C.KuDocument5 pagesCarbonLetter B C.KuMys GenieNo ratings yet
- Corrosion TestsDocument11 pagesCorrosion Testsk21p81No ratings yet
- 1 s2.0 S0010854522004593 Main 2Document58 pages1 s2.0 S0010854522004593 Main 2Mys GenieNo ratings yet
- Recent Advances and New Challenges Two-DimensionalDocument47 pagesRecent Advances and New Challenges Two-DimensionalMys GenieNo ratings yet
- C4RA10227DDocument9 pagesC4RA10227DMys GenieNo ratings yet
- Cu-ZrSe2 ShortDocument7 pagesCu-ZrSe2 ShortMys GenieNo ratings yet
- Energies: Synthesis of The Zno@Zns Nanorod For Lithium-Ion BatteriesDocument8 pagesEnergies: Synthesis of The Zno@Zns Nanorod For Lithium-Ion BatteriesMys GenieNo ratings yet
- Reaction Mechanism of WASSRDocument47 pagesReaction Mechanism of WASSRMys Genie100% (1)
- A Historical Introduction to PhotocatalysisDocument4 pagesA Historical Introduction to PhotocatalysisMys GenieNo ratings yet
- 51 Lithium Intercalation and Crystal Chemistry of Li3VO4Document4 pages51 Lithium Intercalation and Crystal Chemistry of Li3VO4Mys GenieNo ratings yet
- Review Photocatalysis 1 PDFDocument15 pagesReview Photocatalysis 1 PDFMys GenieNo ratings yet
- Sell I Photo Effects Semiconductors Energy EnvironmentDocument43 pagesSell I Photo Effects Semiconductors Energy EnvironmentMys GenieNo ratings yet
- Wend Erich 2016Document33 pagesWend Erich 2016Mys GenieNo ratings yet
- Enhanced Oil Recovery TechniquesDocument18 pagesEnhanced Oil Recovery Techniquesavula43No ratings yet
- Sciencedirect: Co Sorption Characteristics of Various Sorbents in The Bubbling Fluidized-BedDocument5 pagesSciencedirect: Co Sorption Characteristics of Various Sorbents in The Bubbling Fluidized-BedFarah TalibNo ratings yet
- Operating ManualDocument102 pagesOperating ManualLulu ChaniagoNo ratings yet
- Rate Determining StepDocument10 pagesRate Determining Stepxcom100% (1)
- MSC Thesis 1976 Mathematical Model of The SL RN Direct Reduction Process PDFDocument203 pagesMSC Thesis 1976 Mathematical Model of The SL RN Direct Reduction Process PDFJorge EcheverriNo ratings yet
- Status of Miscible Displacement SPE 9992 PADocument12 pagesStatus of Miscible Displacement SPE 9992 PAswaala4realNo ratings yet
- Colligative Properties of SolutionDocument7 pagesColligative Properties of SolutionBianca GeagoniaNo ratings yet
- Improving of Design Parameters of An Industrial Continuous Catalytic Reforming ReactorsDocument15 pagesImproving of Design Parameters of An Industrial Continuous Catalytic Reforming ReactorsArash AbbasiNo ratings yet
- Tutorial DistillationDocument3 pagesTutorial DistillationManu Indivare Nundoolall100% (1)
- 1st Year Chemistry Chapter 2Document3 pages1st Year Chemistry Chapter 2Zeeshan ahmedNo ratings yet
- EKOLYSER Project Single View - Research Energy StorageDocument2 pagesEKOLYSER Project Single View - Research Energy StorageshadyghanemNo ratings yet
- Foam Package Datasheet for Jatiasri Field Collection Station ProjectDocument7 pagesFoam Package Datasheet for Jatiasri Field Collection Station Projectrandi martaNo ratings yet
- N Series Cartridge For Gases/vapors Protection (NIOSH Standard)Document2 pagesN Series Cartridge For Gases/vapors Protection (NIOSH Standard)Diego MartinezNo ratings yet
- PVC Pipes & Fittings: Sanitary Fittings CatalogDocument3 pagesPVC Pipes & Fittings: Sanitary Fittings CatalogDesiree jayne NoriegaNo ratings yet
- Climate Spectator: Landfill Gas Projects Awarded Reverse Auction ContractsDocument2 pagesClimate Spectator: Landfill Gas Projects Awarded Reverse Auction ContractsABC News OnlineNo ratings yet
- Welding Consumables CH-16Document40 pagesWelding Consumables CH-16Qaisir MehmoodNo ratings yet
- Solubility FactorsDocument29 pagesSolubility FactorsEdward Czar PaniqueNo ratings yet
- Refining: Outline Refinery Processes Refining Markets: Capacity, Cost, Investment Optimization of Refinery OperationsDocument49 pagesRefining: Outline Refinery Processes Refining Markets: Capacity, Cost, Investment Optimization of Refinery OperationsJim MacaoNo ratings yet
- Brown Coal Scoping Study - HRL TechDocument51 pagesBrown Coal Scoping Study - HRL TechErsyad FikriansyahNo ratings yet
- Fundamentals of RefiningDocument115 pagesFundamentals of Refiningkelvin84in100% (1)
- Oil and DrillingDocument3 pagesOil and DrillingAmri YogiNo ratings yet
- Visit To LPG PlantDocument6 pagesVisit To LPG PlantOsama HasanNo ratings yet
- What is Membrane Filtration (MFDocument6 pagesWhat is Membrane Filtration (MFAbdulkadir AlbabaNo ratings yet
- CAUSTIC SODA (NaOH) PRODUCTION (19862.en) PDFDocument51 pagesCAUSTIC SODA (NaOH) PRODUCTION (19862.en) PDFAHMEDNo ratings yet
- Chemical Equilibrium Problem Sheet-1Document4 pagesChemical Equilibrium Problem Sheet-1Akash GhoshNo ratings yet
- Peb Evaporation PDFDocument23 pagesPeb Evaporation PDFmessy munozNo ratings yet