Professional Documents
Culture Documents
1.2 The Structure of An Atom - Notebook October 02, 2014
Uploaded by
•θRINEA CHAN•Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1.2 The Structure of An Atom - Notebook October 02, 2014
Uploaded by
•θRINEA CHAN•Copyright:
Available Formats
1.
2 The structure of an atom.notebook October 02, 2014
What is in an atom?
amu = atomic mass unit = 1.66 x 10-27 kg
We use the atomic mass unit to avoid having to deal with very small numbers and it allows
us to describe the mass of a proton and neutron as 1, and an electron as effectively 0.
Different elements have different numbers of protons,
neutrons and electrons. We describe the number of subatomic
particles in an atom using the nuclear symbol:
A
Z
x
Chemical symbol - X
The atomic number - Z
Describes the number of protons in the atom.
Each element has a specific number of protons.
In a neutral atom, we will always have the same number of
electrons as protons (so the charges cancel out).
The mass number - A
Describes the total number of protons and neutrons in the
atom.
Most elements have isotopes which are atoms with the same number of protons but
a different number of neutrons.
Why are each of the examples below hydrogen atoms and how many subatomic
particles are in each of them?
Isotopes have the same number or electrons so have the same chemical
properties but can have different physical properties such as melting point and
boiling point.
(*physical properties can be changed without changing the chemical
composition of the element)
You might also like
- Chemistry FactsheetsDocument415 pagesChemistry FactsheetsAmbrose Aaron DavidNo ratings yet
- File 2657Document6 pagesFile 2657Alexandra LupuNo ratings yet
- Unit 1 Principles of Chemistry: Atomic StructureDocument10 pagesUnit 1 Principles of Chemistry: Atomic StructureKhin Yadanar KyawNo ratings yet
- STPM Sem 1 Introductory Class Notes 2020Document12 pagesSTPM Sem 1 Introductory Class Notes 2020Johnson116No ratings yet
- 1.1. A Simple Model of The Atom, Symbols, Relative Atomic Mass, Electronic Charge and Isotopes PDFDocument4 pages1.1. A Simple Model of The Atom, Symbols, Relative Atomic Mass, Electronic Charge and Isotopes PDFUloko ChristopherNo ratings yet
- 1.1 Atoms and MoleculesDocument54 pages1.1 Atoms and MoleculesAbdullah AhmadNo ratings yet
- Chemie2023 Erdem Gerel UchralDocument21 pagesChemie2023 Erdem Gerel UchralUchral ErkhembayarNo ratings yet
- Atomic StructureDocument162 pagesAtomic StructurevaughanNo ratings yet
- Introduction To Chemistry: Class ObjectivesDocument13 pagesIntroduction To Chemistry: Class ObjectivesSebastian VillegasNo ratings yet
- Unit 2 Notes - Teacher 2Document13 pagesUnit 2 Notes - Teacher 2noNo ratings yet
- Campbell Lecture Notes Chemistry of LifeDocument42 pagesCampbell Lecture Notes Chemistry of LifeSophia Andrei VillalunaNo ratings yet
- Anaphy Chapt 2Document8 pagesAnaphy Chapt 2crptzxraffNo ratings yet
- CHM 092 CHAPTER 1 - Matter &stoichiometryDocument128 pagesCHM 092 CHAPTER 1 - Matter &stoichiometryAisyah NadhirahNo ratings yet
- Chapter 2Document48 pagesChapter 2lelouchali1234No ratings yet
- Nucleus and Elementary Particles: Lesson OneDocument9 pagesNucleus and Elementary Particles: Lesson Onemohy711No ratings yet
- Atoms: 1. Atomic StructureDocument7 pagesAtoms: 1. Atomic Structurecherry shane abanesNo ratings yet
- Chapter 3 Atomic Structure and Periodic TableDocument12 pagesChapter 3 Atomic Structure and Periodic TableAmmar RizwanNo ratings yet
- Chem NotesDocument54 pagesChem Notes42069420zNo ratings yet
- Atoms, Isotopes, Ions, and Molecules: Key PointsDocument28 pagesAtoms, Isotopes, Ions, and Molecules: Key PointsCandyAnonymousNo ratings yet
- 3.1. Atomic Structure and The Periodic TableDocument3 pages3.1. Atomic Structure and The Periodic Tableahmed5030 ahmed5030No ratings yet
- Unit 1: The Atomic Structure of MatterDocument3 pagesUnit 1: The Atomic Structure of MatterDanyel Rodriguez RomeraNo ratings yet
- Chapter 5 - Atomic StructureDocument2 pagesChapter 5 - Atomic StructureMahad AsimNo ratings yet
- 1.1 Atoms and MoleculesDocument60 pages1.1 Atoms and MoleculesMOHAMAD FIRDAUS BIN HARUN KM-PensyarahNo ratings yet
- Atomic Structure and Chemical Bonding: Model of An AtomDocument9 pagesAtomic Structure and Chemical Bonding: Model of An AtomSoumya Ranjan SahooNo ratings yet
- Chap 03Document22 pagesChap 03AmandaNo ratings yet
- Isotopes-General ChemistryDocument28 pagesIsotopes-General Chemistry7assan1300No ratings yet
- 929-Mass Numbers Inc Isotopes PresentationDocument18 pages929-Mass Numbers Inc Isotopes PresentationB.Ed. Wing SundargarhNo ratings yet
- Chapter 22 - Physics - Coordinated Science - IGCSE CambridgeDocument154 pagesChapter 22 - Physics - Coordinated Science - IGCSE CambridgeAlvin DuaneNo ratings yet
- Objective: Topic:ISOTOPESDocument11 pagesObjective: Topic:ISOTOPESMD. ARIFUL ISLAMNo ratings yet
- Make The Grade As and A2 Chemistry Chemistry Revision Guide Edexcel As A2 Modular Nelson Advanced ScienceDocument147 pagesMake The Grade As and A2 Chemistry Chemistry Revision Guide Edexcel As A2 Modular Nelson Advanced Scienceareyouthere92100% (3)
- The Structure of The Atom - Boundless ChemistryDocument13 pagesThe Structure of The Atom - Boundless ChemistrySheena Shane CantelaNo ratings yet
- Radioactivity-General ChemistryDocument26 pagesRadioactivity-General Chemistry7assan1300No ratings yet
- Lab #7 - Build An AtomDocument3 pagesLab #7 - Build An AtomzoritaworkmanNo ratings yet
- 1.1 CchemDocument1 page1.1 CchemcallumNo ratings yet
- II. ATOMS, MOLECULES and IONSDocument18 pagesII. ATOMS, MOLECULES and IONSHania ABDULNo ratings yet
- CP 17 Lab 2 Build An Atom PhET SimulationDocument4 pagesCP 17 Lab 2 Build An Atom PhET SimulationAlexandra. NNo ratings yet
- 7b Modern Atomic Theory, Subatomic Particles and Structure of AtomDocument32 pages7b Modern Atomic Theory, Subatomic Particles and Structure of AtomMaaz WaseemNo ratings yet
- Chapter 2 Gen ChemDocument10 pagesChapter 2 Gen ChemJennifer MalunaoNo ratings yet
- Atomic Structure: 2.1 The AtomDocument22 pagesAtomic Structure: 2.1 The AtomMelanny Johemy Jordán VásquezNo ratings yet
- Atomic Structure Notes - LectureDocument19 pagesAtomic Structure Notes - LecturealexandremoutonNo ratings yet
- Eastern Samar National Comprehensive High School Chemistry 1Document3 pagesEastern Samar National Comprehensive High School Chemistry 1Isaac PiaoNo ratings yet
- Atomic StructureDocument50 pagesAtomic StructureangelynNo ratings yet
- Atomic Structure & The Periodic TableDocument25 pagesAtomic Structure & The Periodic Tablestan AB6IXNo ratings yet
- Atomic Structure and Periodic TableDocument67 pagesAtomic Structure and Periodic Tablelsllsl9471No ratings yet
- Chapter 2 - Lecture 1 F22Document16 pagesChapter 2 - Lecture 1 F22Ali AtwiNo ratings yet
- (TC) C2 Atomic Structure and Electronic ConfigurationDocument5 pages(TC) C2 Atomic Structure and Electronic Configurationthanat amornratchnondNo ratings yet
- FLGX113 Su2.1Document40 pagesFLGX113 Su2.1Jason Van Den HeeverNo ratings yet
- Bioenergetics BasicsDocument16 pagesBioenergetics Basicsdwr135No ratings yet
- Chapter 1: Matter 1.1 Atoms and Molecules: Packed in A Small NucleusDocument35 pagesChapter 1: Matter 1.1 Atoms and Molecules: Packed in A Small NucleusSupia NazmaNo ratings yet
- Atom, Molecule and Stoichiometry 2023 OnlineDocument10 pagesAtom, Molecule and Stoichiometry 2023 OnlineGan Ee HengNo ratings yet
- Concepts of Nuclear Medicine Volume I: Concepts of Nuclear Medicine, #1From EverandConcepts of Nuclear Medicine Volume I: Concepts of Nuclear Medicine, #1No ratings yet
- A-Level Chemistry Revision: Cheeky Revision ShortcutsFrom EverandA-Level Chemistry Revision: Cheeky Revision ShortcutsRating: 4 out of 5 stars4/5 (5)
- Everything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksFrom EverandEverything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksNo ratings yet
- Your Journey To The Basics Of Quantum Realm Volume II: Your Journey to The Basics Of Quantum Realm, #2From EverandYour Journey To The Basics Of Quantum Realm Volume II: Your Journey to The Basics Of Quantum Realm, #2Rating: 5 out of 5 stars5/5 (1)
- Jeffrey Bezos (Ingles)Document3 pagesJeffrey Bezos (Ingles)•θRINEA CHAN•No ratings yet
- Hipothesis Del Arcoiris (Ingles)Document3 pagesHipothesis Del Arcoiris (Ingles)•θRINEA CHAN•No ratings yet
- Amazon (Jeff Bezos) Presentacion (Ingles)Document10 pagesAmazon (Jeff Bezos) Presentacion (Ingles)•θRINEA CHAN•No ratings yet
- UNIT 3: Pure Substances and Mixtures 2º ESO: Susana Morales BernalDocument41 pagesUNIT 3: Pure Substances and Mixtures 2º ESO: Susana Morales Bernal•θRINEA CHAN•No ratings yet
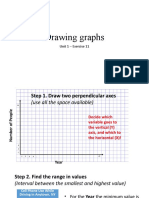
- Drawing Graphs: Unit 1 - Exercise 11Document6 pagesDrawing Graphs: Unit 1 - Exercise 11•θRINEA CHAN•No ratings yet
- They Are Base or Derived:: A) 5 Ms B) 2 Ha C) 1 250 NM D) 0.0032 MolDocument1 pageThey Are Base or Derived:: A) 5 Ms B) 2 Ha C) 1 250 NM D) 0.0032 Mol•θRINEA CHAN•No ratings yet
- Exercise - 1: Basic Objective Questions: Ionic BondsDocument7 pagesExercise - 1: Basic Objective Questions: Ionic BondsNavita RajgariaNo ratings yet
- Intrinsic and Extrinsic N Type P TypeDocument4 pagesIntrinsic and Extrinsic N Type P TypeDinesh VelNo ratings yet
- Hybridization: DR Amalina Mohd TajuddinDocument27 pagesHybridization: DR Amalina Mohd TajuddinNazmi LatifNo ratings yet
- Physical Science Module 2 2Document7 pagesPhysical Science Module 2 2Khrisha TindoyNo ratings yet
- 16a Periodic Table GeneralDocument13 pages16a Periodic Table GeneralfarahNo ratings yet
- Intermediate Latin GrammarDocument621 pagesIntermediate Latin GrammarMichael ShortNo ratings yet
- Anachem NotesDocument1 pageAnachem NoteshNo ratings yet
- Mass Spectrometry PPT by KusumDocument23 pagesMass Spectrometry PPT by KusumKusum AhlawatNo ratings yet
- Atomic Structure ChemistryDocument143 pagesAtomic Structure ChemistryYoshitha Kuntumalla100% (1)
- ULAS Sci8Q3 WK 5 6Document10 pagesULAS Sci8Q3 WK 5 6Joan MarieNo ratings yet
- X-Ray Fluorescence SpectrosDocument4 pagesX-Ray Fluorescence SpectroskousikkumaarNo ratings yet
- Week2Quiz1 PDFDocument1 pageWeek2Quiz1 PDFSanam NiazNo ratings yet
- CHEM340 Tut AAS With AnswersDocument4 pagesCHEM340 Tut AAS With AnswersAlex Tan100% (2)
- Emision Lines SDSSDocument3 pagesEmision Lines SDSSRamses Jerez NicurcarNo ratings yet
- Energetics of Jahn Teller EffectDocument8 pagesEnergetics of Jahn Teller EffectFalak NazNo ratings yet
- Q2 Week 1 Copy 1Document5 pagesQ2 Week 1 Copy 1Roberto Misola Jr.No ratings yet
- 03 Atomic StructureDocument68 pages03 Atomic StructureSUJATA SAHUNo ratings yet
- Waters LC-MS PrimerDocument44 pagesWaters LC-MS PrimerKiran ChokshiNo ratings yet
- Chapter 2 Chemistry For Engineers Final Module 2Document26 pagesChapter 2 Chemistry For Engineers Final Module 2Alex Jr. Rosadiño C.No ratings yet
- Question Bank On Electronic Conf.Document6 pagesQuestion Bank On Electronic Conf.Harsh TyagiNo ratings yet
- Dual Nature NCERT NotesDocument14 pagesDual Nature NCERT Notessaifkhan252006No ratings yet
- Band Theory of MetalsDocument3 pagesBand Theory of MetalsShirley DasNo ratings yet
- Nuclear ChemistryDocument14 pagesNuclear ChemistryGoldèn DawnNo ratings yet
- InstrumentationDocument4 pagesInstrumentationRainneTayNo ratings yet
- Energie of Helium LikeDocument10 pagesEnergie of Helium LikeYant HArdy WaeNo ratings yet
- Whole Pattern Fitting and Rietveld Refinement: Sr0.33Mnla0.67O2.91 (Xs (?) 876 (151), LC - 0.936)Document1 pageWhole Pattern Fitting and Rietveld Refinement: Sr0.33Mnla0.67O2.91 (Xs (?) 876 (151), LC - 0.936)baokeliNo ratings yet
- Electronic Structure of MatterDocument14 pagesElectronic Structure of MatterSharlene GonzagaNo ratings yet
- The Periodic Table, Electron Shells, and OrbitalsDocument13 pagesThe Periodic Table, Electron Shells, and OrbitalsCandyAnonymousNo ratings yet
- Nuclear Chemistry Problems-1Document4 pagesNuclear Chemistry Problems-1Marques CatheyNo ratings yet
- AssiDocument3 pagesAssiScott BoothNo ratings yet