Professional Documents
Culture Documents
HalfCellPoten Table
Uploaded by
Jeisson David Sanchez PInzon0 ratings0% found this document useful (0 votes)
7 views1 pageThis document lists standard half-cell potentials for various reduction half-reactions in aqueous solution at 25°C. The half-reactions are organized from most positive to most negative potential. Fluorine gas has the most positive potential of +2.87 V, while lithium has the most negative potential of -3.045 V. Standard potentials provide a measure of the tendency of a half-reaction to occur under standard state conditions.
Original Description:
Original Title
HalfCellPoten_table
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document lists standard half-cell potentials for various reduction half-reactions in aqueous solution at 25°C. The half-reactions are organized from most positive to most negative potential. Fluorine gas has the most positive potential of +2.87 V, while lithium has the most negative potential of -3.045 V. Standard potentials provide a measure of the tendency of a half-reaction to occur under standard state conditions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
7 views1 pageHalfCellPoten Table
Uploaded by
Jeisson David Sanchez PInzonThis document lists standard half-cell potentials for various reduction half-reactions in aqueous solution at 25°C. The half-reactions are organized from most positive to most negative potential. Fluorine gas has the most positive potential of +2.87 V, while lithium has the most negative potential of -3.045 V. Standard potentials provide a measure of the tendency of a half-reaction to occur under standard state conditions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
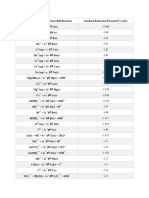
Standard Half-Cell Potentials in Aqueous Solution at 25 ˚C
Reduction Half-Reaction Half-Cell E˚ (V)
F2(g) + 2 e⁻ → 2 F⁻(aq) F2(g) | F⁻(aq) | Pt +2.87
H2O2(aq) + 2 H+(aq) + 2 e⁻ → 2 H2O(ℓ) H2O2(aq), H+(aq), H2O (ℓ) | Pt +1.763
PbO2(s) + SO42-(aq) + 4 H+(aq) + 2 e⁻ → PbSO4(s) + 2 H2O(ℓ) PbO2(s) | SO42-(aq), H+(aq) | PbSO4(s) | Pb +1.690
Au3+(aq) + 3 e⁻ → Au(s) Au3+(aq) | Au(s) +1.52
MnO4⁻(aq) + 8 H+(aq) + 5 e⁻ → Mn2+(aq) + 4 H2O(ℓ) MnO4⁻(aq), H+(aq), Mn2+(aq) | Pt +1.51
Cr2O72-(aq) + 14 H+(aq) + 6 e⁻ → 2 Cr3+(aq) + 7 H2O(ℓ) Cr2O72-(aq), H+(aq), Cr3+(aq) | Pt +1.36
Cl2(g) + 2 e⁻ → 2 Cl⁻(aq) Cl2(g) | Cl⁻(aq) | Pt +1.358
O2(g) + 4 H+(aq) + 4 e⁻ → 2 H2O(ℓ) O2(g) | H+(aq) | Pt +1.229
Br2(ℓ) + 2 e⁻ → 2 Br⁻(aq) Br2(ℓ) | Br⁻(aq) | Pt +1.066
NO3⁻(aq) + 4 H+(aq) + 3 e⁻ → NO(g) + 2 H2O(ℓ) NO3⁻(aq), H+(aq) | NO(g) | Pt +0.96
OCl⁻(aq) + H2O(ℓ) + 2 e⁻ → Cl⁻(aq) + 2 OH⁻(aq) OCl⁻(aq), Cl⁻(aq), OH⁻(aq) | Pt +0.89
Hg2+(aq) + 2 e⁻ → Hg(ℓ) Hg2+(aq) | Hg(ℓ) +0.8535
Ag+(aq) + e⁻ → Ag(s) Ag+(aq) | Ag(s) +0.7991
Hg22+(aq) + 2 e⁻ → 2 Hg(ℓ) Hg22+(aq) | Hg(ℓ) +0.7960
Fe3+(aq) + e⁻ → Fe2+(aq) Fe3+(aq), Fe2+(aq) | Pt +0.771
I2(s) + 2 e⁻ → 2 I⁻(aq) I2(s) | I⁻(aq) | Pt +0.535
O2(g) + 2 H2O(ℓ) + 4 e⁻ → 4 OH⁻(aq) O2(g) | OH⁻(aq) | Pt +0.401
Cu2+(aq) + 2 e⁻ → Cu(s) Cu2+(aq) | Cu(s) +0.340
Sn4+(aq) + 2 e⁻ → Sn2+(aq) Sn4+(aq), Sn2+(aq) | Pt +0.15
2 H+(aq) + 2 e⁻ → H2(g) H+(aq) | H2(g) | Pt 0
Sn2+(aq) + 2 e⁻ → Sn(s) Sn2+(aq) | Sn(s) -0.1375
Ni2+(aq) + 2 e⁻ → Ni(s) Ni2+(aq) | Ni(s) -0.25
PbSO4(s) + 2 e⁻ → Pb(s) + SO42-(aq) PbSO4(s) | SO42-(aq) | Pb(s) -0.3505
Cd2+(aq) + 2 e⁻ → Cd(s) Cd2+(aq) | Cd(s) -0.403
Fe2+(aq) + 2 e⁻ → Fe(s) Fe2+(aq) | Fe(s) -0.44
Zn2+(aq) + 2 e⁻ → Zn(s) Zn2+(aq) | Zn(s) -0.763
2 H2O(ℓ) + 2 e⁻ → H2(g) + 2 OH⁻(aq) H2O (ℓ), OH⁻(aq) | H2(g) | Pt -0.8277
Al3+(aq) + 3 e⁻ → Al(s) Al3+(aq) | Al(s) -1.676
Mg2+(aq) + 2 e⁻ → Mg(s) Mg2+(aq) | Mg(s) -2.356
Na+(aq) + e⁻ → Na(s) Na+(aq) | Na(s) -2.714
K+(aq) + e⁻ → K(s) K+(aq) | K(s) -2.925
Li+(aq) + e⁻ → Li(s) Li+(aq) | Li(s) -3.045
You might also like
- SRP Table Chem DataDocument1 pageSRP Table Chem Dataapi-222503660No ratings yet
- Chapter 3 Oxidation ReductionDocument68 pagesChapter 3 Oxidation Reductionlong.vuongbz188No ratings yet
- SOA and SRA TableDocument1 pageSOA and SRA TableAhhhhhhhhhhhNo ratings yet
- DebateDocument3 pagesDebatebbangeles1No ratings yet
- Chemistry Stage 2 and 3 Data Sheet 2010Document4 pagesChemistry Stage 2 and 3 Data Sheet 2010Edy LiewNo ratings yet
- Standard Electrode Potential SeriesDocument1 pageStandard Electrode Potential SeriesWONG KEE PING MoeNo ratings yet
- Standard Electrode Potentials in Aqueous Solution at 25°C: TablesDocument2 pagesStandard Electrode Potentials in Aqueous Solution at 25°C: TablesLouie G NavaltaNo ratings yet
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFalbi veshiNo ratings yet
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFAlexander Salado IbrahimNo ratings yet
- Standardreductionpotentials PDFDocument1 pageStandardreductionpotentials PDFBadrus SyamsiNo ratings yet
- 233 SolutionsDocument11 pages233 Solutionsestellasr00No ratings yet
- Chapter 4 Oxidation-ReductionDocument68 pagesChapter 4 Oxidation-ReductionPHƯƠNG ĐẶNG YẾNNo ratings yet
- Sap 5Document22 pagesSap 5reza noviyantiNo ratings yet
- Standard Reduction PotentialsDocument3 pagesStandard Reduction PotentialsjaverfrivNo ratings yet
- Extract 10 PagesDocument10 pagesExtract 10 PageskuoklukeNo ratings yet
- CHEM1 Datasheet May 2020Document4 pagesCHEM1 Datasheet May 2020Miku HatsuneNo ratings yet
- Edexecel IAL Lesson 1Document20 pagesEdexecel IAL Lesson 1Pevin De silvaNo ratings yet
- Reactions For Preparation of A Few GasesDocument1 pageReactions For Preparation of A Few Gasesraamki_99No ratings yet
- Chem 2Document8 pagesChem 22021302095No ratings yet
- Standard Reduction Potentials Data Extended PDFDocument2 pagesStandard Reduction Potentials Data Extended PDFAceNo ratings yet
- Standard Reduction PotentialDocument7 pagesStandard Reduction Potentialyoyotoonzone1No ratings yet
- Appendix EDocument1 pageAppendix EYrhon IbanezNo ratings yet
- Silo - Tips 2 Write The Chemical Formulas of The Products and Balance The Following Spontaneous ReactionsDocument40 pagesSilo - Tips 2 Write The Chemical Formulas of The Products and Balance The Following Spontaneous ReactionsAkash BhoiNo ratings yet
- Reduction Half-Reaction E (V) : Neutral or Acid SolutionDocument3 pagesReduction Half-Reaction E (V) : Neutral or Acid SolutionEric FernandoNo ratings yet
- STD Electrode PontentialsDocument3 pagesSTD Electrode PontentialsVishal PamnaniNo ratings yet
- Chemical Reactions Class XDocument5 pagesChemical Reactions Class Xaprajita royNo ratings yet
- Lesson Plan 5Document15 pagesLesson Plan 5Gusty DyanoNo ratings yet
- Problems For Balancing of Redox ReactionsDocument1 pageProblems For Balancing of Redox ReactionsUtsavNo ratings yet
- Chem16 Experiment # 1: Chemical Changes Post-Lab Discussion: 3 2 (Aq) (Aq) 2 (S) 3 (Aq) 2+ (Aq) - (Aq) 2 (S)Document1 pageChem16 Experiment # 1: Chemical Changes Post-Lab Discussion: 3 2 (Aq) (Aq) 2 (S) 3 (Aq) 2+ (Aq) - (Aq) 2 (S)DiyanikaNo ratings yet
- Balancing Equations Worksheet Key: ZN (S) + 2 AgnoDocument1 pageBalancing Equations Worksheet Key: ZN (S) + 2 AgnoIgnacio Jr. PaguyoNo ratings yet
- IONIC EQUATIONS AnswersDocument1 pageIONIC EQUATIONS AnswersAlex noslenNo ratings yet
- New Microsoft Word DocumentDocument4 pagesNew Microsoft Word DocumentdalvishreyhansNo ratings yet
- Equation AnswersDocument12 pagesEquation AnswersMark CalawayNo ratings yet
- ElectrodeDocument2 pagesElectrodeThatcher PanchoNo ratings yet
- Standard Reduction (Electrode) PotentialsDocument2 pagesStandard Reduction (Electrode) Potentialsbackch9011No ratings yet
- Balancing Redox Reactions WorksheetDocument2 pagesBalancing Redox Reactions Worksheetsai sreerama mNo ratings yet
- Salts FormationDocument19 pagesSalts FormationUrwa Abdul MannanNo ratings yet
- CH 11 - Writing - Chemical - Equation - 1 - AnsDocument2 pagesCH 11 - Writing - Chemical - Equation - 1 - AnsOlivia LinNo ratings yet
- Chemical EquationsDocument5 pagesChemical EquationsShweta DharNo ratings yet
- Chemistry Form 4 Lesson 12Document8 pagesChemistry Form 4 Lesson 12Sakinah SaadNo ratings yet
- Chapter 5 Answers Practice Examples: ReductionDocument7 pagesChapter 5 Answers Practice Examples: ReductionEmre Enes EdizNo ratings yet
- Chemical Reactions and Equations WS-1Document2 pagesChemical Reactions and Equations WS-1Naman SinghNo ratings yet
- Chemical Reactions in Aqueous SolutionDocument5 pagesChemical Reactions in Aqueous Solutioniam_crazii_4_mhe100% (2)
- Chapter-Wise Important Chemical Reactions For Class 10Document9 pagesChapter-Wise Important Chemical Reactions For Class 10Manish SainNo ratings yet
- Standard Electrode PotentialDocument11 pagesStandard Electrode PotentialRSLNo ratings yet
- Chemical Reactions: Processes and TypesDocument38 pagesChemical Reactions: Processes and TypesSlay SacedaNo ratings yet
- Standard Reduction PotentialsDocument1 pageStandard Reduction PotentialsAnonymous s4HW3TX0IHNo ratings yet
- Standard Reduction PotentialDocument1 pageStandard Reduction PotentialghanifdkdNo ratings yet
- Standardreductionpotential PDFDocument1 pageStandardreductionpotential PDFShiizan123No ratings yet
- Standardreductionpotentials PDFDocument1 pageStandardreductionpotentials PDFEsat GoceriNo ratings yet
- Chemistry Databook WDocument24 pagesChemistry Databook Wdaemperor216No ratings yet
- Reaksi Pemisahan KationDocument2 pagesReaksi Pemisahan KationAnisa Nursella TrunodikromoNo ratings yet
- Cations LabDocument4 pagesCations LabKayshalee BlackburnNo ratings yet
- As Equations: Lithium Monoxide (Contains O) Sodium Peroxide (Contains O) Potassium Superoxide (Contains O)Document4 pagesAs Equations: Lithium Monoxide (Contains O) Sodium Peroxide (Contains O) Potassium Superoxide (Contains O)arcmikeNo ratings yet
- Standard Reduction PotentialsDocument5 pagesStandard Reduction PotentialsnathaloaNo ratings yet
- Ex Cell NotationDocument2 pagesEx Cell NotationveemueNo ratings yet
- ReaksiDocument2 pagesReaksiherna watiNo ratings yet
- ch12 Odd PDFDocument37 pagesch12 Odd PDFmecsolNo ratings yet
- Net Ionic Equations With AnswersDocument12 pagesNet Ionic Equations With Answersenileuqcaj100% (1)
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Agip Eni Alaria-2 - 3 - 7Document2 pagesAgip Eni Alaria-2 - 3 - 7Andre WantoNo ratings yet
- Cutting FluidDocument8 pagesCutting FluidDevarakonda KondayyaNo ratings yet
- Non-Destructive Tests On Eco-Friendly Anti-Corrosion Paint: September 2017Document10 pagesNon-Destructive Tests On Eco-Friendly Anti-Corrosion Paint: September 2017nanoNo ratings yet
- DT Gen 10001Document63 pagesDT Gen 10001KelvinNo ratings yet
- AND From AND: FOR Rhodium, For Their GravimetricDocument13 pagesAND From AND: FOR Rhodium, For Their GravimetricshahinNo ratings yet
- Effect of Heat Treatment On Microstructure and Mechanical Behaviours of 18ni-300 Maraging Steel Manufactured by Selective Laser MeltingDocument11 pagesEffect of Heat Treatment On Microstructure and Mechanical Behaviours of 18ni-300 Maraging Steel Manufactured by Selective Laser MeltingHasan TaşNo ratings yet
- CTO FullDocument98 pagesCTO FullRamesh Babu100% (1)
- Inspection and Test Plan No 826 Vendor Qualification For Hardfacing Overlay of Seat Rings and DiscsDocument3 pagesInspection and Test Plan No 826 Vendor Qualification For Hardfacing Overlay of Seat Rings and DiscsGohilakrishnan ThiagarajanNo ratings yet
- Transcript of MarksDocument2 pagesTranscript of MarksKiranPadman50% (2)
- Fully Developed Flow Between Two Parallel PlatesDocument7 pagesFully Developed Flow Between Two Parallel PlatesKantharaj ChinnappaNo ratings yet
- I - Grades & Materilas InfoDocument32 pagesI - Grades & Materilas InfoEswara ReddyNo ratings yet
- Lec8 RTMDocument31 pagesLec8 RTMmoonrock1No ratings yet
- Qualitative ChemistryDocument74 pagesQualitative Chemistryবিশ্বস্ত মিথ্যাবাদীNo ratings yet
- Fluoride Toxicity SeminarDocument27 pagesFluoride Toxicity SeminarNikhil BhandariNo ratings yet
- Same 023Document2 pagesSame 023amardeepbediNo ratings yet
- Design of Portal Frames - NotesDocument32 pagesDesign of Portal Frames - NotesAdi Andriescu0% (1)
- Check Valve (Swagelok) MS-01-176 PDFDocument16 pagesCheck Valve (Swagelok) MS-01-176 PDFIsmailIbrahimNo ratings yet
- History of Medicine, Alexander WilderDocument994 pagesHistory of Medicine, Alexander Wilderchesterj2100% (1)
- Chromatographyvolume 1Document99 pagesChromatographyvolume 1JOSE R. LEALNo ratings yet
- Introduction To The Problems Surrounding Garment TextilesDocument30 pagesIntroduction To The Problems Surrounding Garment TextilesFathi Mustafa100% (1)
- Thermal Expansion Settings in GleebleDocument4 pagesThermal Expansion Settings in GleeblePranav TripathiNo ratings yet
- Que BankDocument12 pagesQue BankAbhishek VishwakarmaNo ratings yet
- CO2 Absorption by Ionic LiquidsDocument52 pagesCO2 Absorption by Ionic LiquidsMohd. Belal HaiderNo ratings yet
- Regulatory AffairsDocument14 pagesRegulatory AffairsSiddarth Reddy100% (2)
- Chemical Food SafetyDocument297 pagesChemical Food SafetyqtrystNo ratings yet
- Scaling of MosfetDocument40 pagesScaling of MosfetSaumitra TripathiNo ratings yet
- Methods and ProceduresDocument9 pagesMethods and ProceduresQuebec GC RPhNo ratings yet
- Almex Conveyor Belt Training Manual (Glossary of Terms Section)Document27 pagesAlmex Conveyor Belt Training Manual (Glossary of Terms Section)Luis FloresNo ratings yet
- Wl2000it Brochure ENGDocument2 pagesWl2000it Brochure ENGSantos RodriguezNo ratings yet
- Ir Pd15x-Xxx-Xxx-EnDocument8 pagesIr Pd15x-Xxx-Xxx-Enlobo7012No ratings yet