Professional Documents
Culture Documents
Alcohols
Uploaded by
Suhaan GargOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alcohols
Uploaded by
Suhaan GargCopyright:
Available Formats
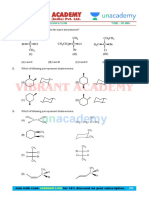
AMITY INSTITUTE FOR COMPETITIVE EXAMINATIONS
WORK SHEET SANJEEV AWASTHI
CHEMISTRY ALCOHOLS
Choose the correct answer
1. Chromic acid oxidations are believed no proceed through a chromate ester intermeidate. In the oxidation of
ethan-1-ol, the intermeidate which form is
O O
OH O
(a) H C Cr (b) H C Cr
3 O 3 O
O O
O O
O
(c) Cr (d) H C Cr
H3C O OH 3 O
O
2. Compounds ‘A’, ‘B’ and ‘C’ are isomeric alcoholswith the formula C5H12O, ‘A’ react with chromic acid solution,
B forming an acid. The three isomers react with HBr with decreasing relative rates:
C A B, all giving the same C5H11Br in varying yields. ‘A’ alone can be oxidised by I2, OH–.
Consider the following molecules:
OH OH
OH
OH H3C H3C
I. H3C II. III. IV.
H3C CH3 CH3 OH CH3 CH3
H3C CH3 OH
V. VI.
H3C OH H3C OH
(a) A – II, B – I, C – IV (b) A – V, B – VI, C – IV
(c) A – III, B – II, C – IV (d) A – V, B – III, C – IV
OH
NaOH CH3 OTs
(1 eq)
Product
3.
OH Cl
The predominant product is
OH OH CH3 CH3
O O
(a) (b) (c) (d)
O Cl OH O
H3C CH3 OH Cl OH OH
Amity Institute for Competitive Examinations Phones: 011-41888030, 41888031, 41888032
-1-
4. Consider the following reactions:
Br Br Br
OH OH OH
I. II. III.
H3C C CH3 H3C C CH3 H3C C CH3
CH3 CH3 CH3
Choose the correct decreasing rate of epoxidation on treatment with OH–/H2O.
(a) I II III (b) II III I (c) III II I (d) II I III
5. What results when but-I-ene is subjected to the following reaction sequence
I. Cl2, H2O II. NaOH III. H3O+
(a) Meso compound (b) a 1 : 1 mixture of enantiomeric epoxides
(c) a meso diol (d) a 1 : 1 mixture of enantiomeric diols
CH3
(CH3CO)2 O, H AlCl3
A
6. O
Find product ‘A’ is
CH3
OH
(a) OH (b)
O CH3
O CH3
H3C CH3 O
CH3
(c) O (d) OH
O CH3
+
H
7. Reaction I: cis-1,2-dimethyl cyclohexane-1,2-diol A
+
H
Reaction II: trans-1,2-dimethyl cyclohexane-1,2-diol B
Product ‘A’ and ‘B’ are
(a) same as (b) same as
O
O
(c) respectively and (d) respectively and
O
O
O O
Amity Institute for Competitive Examinations Phones: 011-41888030, 41888031, 41888032
-2-
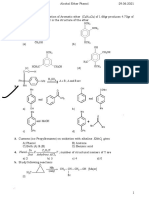
8. Which of he following reaction gives the alcohol which is lower homologue of the reacting substrate?
CH3 Ph
H O ;H
(a) H 2O 2 ;H
(b)
OH
2 2
H3C OH H3C
Ph
H 2O2 ;H
OH H 2 O 2 ;H
(c) (d) OH
H3C Ph
OH KMnO 4 conc. HI
9. A
one. eq. of CH I
C9 H10 O3 C9 H10 O 4
3,4 dihydroxy benzoic acid
3
(It gives intense colour with (HCO3 so luble)
FeCl3 and Positive Tollen's
test)
Starting substrate ‘A’ is
OH OH
OH OMe
(a) (b)
H3C
CH3 O
CH3
OH O
OH O
CH3
(c) O (d)
CH3
O
10. Identify end product of the following:
(i ) D
(a) CH3CH = CH2 A
( ii ) H O 2
(i ) H
(b) CH3CH = CH2 B
(ii ) D 2 O
11. NaOH
+ NBS A B, What are A and B?
12. Identify end product in the following:
3 BH . THF 6 5 C H CO H
(a) CH3CH = CH2 (b) CH3CH = CH2 3
H O /OH 2 2 LiAlH /H 4
(c) 4LiAlH /H
O
13. Identify A and B in the following Hydroboration oxidtion (HBO) reaction:
A, B
Amity Institute for Competitive Examinations Phones: 011-41888030, 41888031, 41888032
-3-
14. Identify the final product of the oxymercuration-demercuration of
(a) (b)
Me
(c) (d)
15. Identify A to D in the following reactions:
3CH MgBr 3 3 BH /THF CH MgBr
D C
H 3O HgSO
4 /H 2SO4
CH3C CH
A
H 2O 2 /OH
B
H 3O
16. Identify A and B in the following reaction:
H 3O
OCH3 + 2CH3CH2MgBr
(a) A
O
O
|| H O
(b) PhCCl + 2 3 B
MgCl
CH 3
|
CO, H 2 , [CoH(CO) 4 ] H2
17. CH 3CCH 2C CH 2 A B
| | 125 , pressure catalyst
CH 3 CH 3
18. What are the products A, B, C, D and E in the following?
NaBH4
O O CH3OH
NaBH4
CH3OH LiAlH4
O
O
O O
OEt
NaBH4 OEt LiAlH4
CH3OH
O O
19. What are the various products in the following?
1. O3 /H 2 O/Zn
2. LiAlH4
Amity Institute for Competitive Examinations Phones: 011-41888030, 41888031, 41888032
-4-
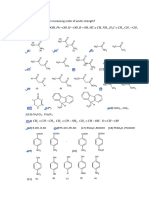
AMITY INSTITUTE FOR COMPETITIVE EXAMINATIONS
WORK SHEET
CHEMISTRY ALCOHOLS
O
Br / h Mg CH3–C–CH3 H
Q.1 CH3–CH=CH2 2
dry ether NH4Cl
The end product in the above sequence of reaction is
(a) CH2=CH–CH2– C=CH2 (b) H2C=CH–CH= C–CH3
CH3 CH3
OH
(c) H2C=CH–CH2– C–CH3 (d) H2C=CH–CH2– CH–CH2–OH
CH3 CH3
Q.2 OH Hg (OAc) 2
the major product is
NaBH 4
O
OH OH OH
(a) (b) (c) (d)
OH
OH
Br2 / CCl 4
Q.3 OH The major product is
O O
(a) Br OH (b) (c) (d)
Br O
Br
Br
18
OH H+
Q.4 The major product is
OH
18
(a) (b) (c) OH (d)
O O18 OH
Q.5 Diols which react with CrO 3 in aqueous H 2SO 4 and yield product that readily undergo
decarboxylation on heating, are
OH
OH
(a) (b) HO (c) HO OH (d)
HO OH OH
Amity Institute for Competitive Examinations Phones: 011-41888030, 41888031, 41888032 1
18
CH2–Cl AgOCOCH H3O+
Q.11 3 A B+ C. The compounds B and C are:
18
O 18 O
CH2–OH CH2–OH 18
(a) + CH3– C –OH (b) + CH3– C – OH
O 18 O
CH2–OH CH2–OH
(c) + CH3– C –OH (d) + CH3– C –OH
Q.12 C (1) LiAlH4 ? The product is
N
(2) H 3O
CH=O
(a) N CH2–NH2 (b) N
(c) N CH=NH (d) N CH2–OH
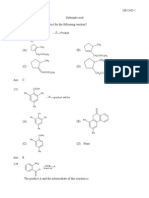
OH
H
Q.13 Ph—CH—C CH ? The product is:
(a) Ph–CH=CH–CH=O (b) Ph–CC–CHO
(c) Ph–CH=C=CH–OH (d) Ph–CC–CH2–OH
Q.14 Boiling point of cis-1,3-cyclohexanediol is................ than cis-1,4-cyclohexanediol
(a) lesser (b) greater (c) equal (d) can’t be predicted
Q.15 Cineole is the chief component of eucalyptus oil, it has, molecular formula C10H18O and
contains no double bond or triple bond. It reacts with HCl to give:
CH3
H3C–C–Cl
Cineole HCl
Cl
The structure of cineole is: CH3
CH3 H3C
H3C–C–OH C=CH2 O O
(a) (b) (c) (d)
OH OH CH
CH3 CH3 H3C CH3
Amity Institute for Competitive Examinations Phones: 011-41888030, 41888031, 41888032 3
HO OH
H
Q.21 ? The major product is:
O
CHO
(a) (b)
OH
O
(c) (d)
O
–
OH Sn / HCl NaNO / HCl
Q.22 + CH3NO2 A B 2
C. The product C is
CH2–NH2
OH O OH
OH CH2–OH
(a) (b) (c) (d)
OH
AgNO
Q.23 CH3–C–CH2–I 3 ? The product is:
Ph
O
(a) CH3–C–CH2 (b) CH3–CH2–C
O
OH Ph
(c) CH 3–C–CH3 (d) CH3–CH–CH2
Ph
CH3CH3
H
Q.24 CH3—C—C— CH3 ? The product is:
O
CH3 O CH3
(a) CH3—CH—C—CH3 (b) CH3—C—C—CH3
OH CH3 CH3
CH3CH3
(c) CH3—C C—CH3 (d) CH3—C—C—CH3
CH3 CH3 OH H
Amity Institute for Competitive Examinations Phones: 011-41888030, 41888031, 41888032 5
You might also like
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- Org 1 PDFDocument4 pagesOrg 1 PDFTanmay KumarNo ratings yet
- OH A, A Is: : CHN 1 EqvDocument4 pagesOH A, A Is: : CHN 1 EqvAtharva GanjuNo ratings yet
- JEE Advanced Alcohols, Phenols and Ethers Important QuestionsDocument17 pagesJEE Advanced Alcohols, Phenols and Ethers Important QuestionsPiyush kumarNo ratings yet
- Alcohol, Ether & PhenolDocument8 pagesAlcohol, Ether & Phenolshashwat.gupta.707No ratings yet
- Isomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomersDocument11 pagesIsomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomerskamalNo ratings yet
- Chemistry PracticeDocument13 pagesChemistry PracticeSiddharth KrishnamurthyNo ratings yet
- PDFDocument30 pagesPDFAlok RanjanNo ratings yet
- DPP Optical+Isomer 4Document2 pagesDPP Optical+Isomer 4Shivam RoyNo ratings yet
- Quiz-Alcohol Ether & Phenols-Rsk - RGVDocument6 pagesQuiz-Alcohol Ether & Phenols-Rsk - RGVAtharva GanjuNo ratings yet
- JEE Main Organic Compound Containing Halogens Important QuestionsDocument15 pagesJEE Main Organic Compound Containing Halogens Important QuestionsRuchitha VNo ratings yet
- Incom. Sr. 29.06.2021 AssignmentDocument5 pagesIncom. Sr. 29.06.2021 AssignmentSrikar SatyaNo ratings yet
- The Carbonyl Compound-3Document3 pagesThe Carbonyl Compound-3devender singhNo ratings yet
- Ape Assignment 3Document7 pagesApe Assignment 3Atharva KulkarniNo ratings yet
- Ejercicios de Alcoholes y EteresDocument1 pageEjercicios de Alcoholes y EteresMaria Jose Duque AngelNo ratings yet
- Chemistry Paper - Ii Solution (Code 3)Document5 pagesChemistry Paper - Ii Solution (Code 3)kolodoloNo ratings yet
- Class Test-4 - Without AnsDocument5 pagesClass Test-4 - Without AnsshouryatrialNo ratings yet
- Alcohol, Ether and PhenolDocument4 pagesAlcohol, Ether and PhenolKushagra SrivastavaNo ratings yet
- Study HourDocument11 pagesStudy HourG SATHVIKNo ratings yet
- Alcohols Phenols EtherDocument55 pagesAlcohols Phenols EtherAnanya AgrawalNo ratings yet
- 2008Document7 pages2008prakhar vishwakarmaNo ratings yet
- Rapid Crash Course: Single CorrectDocument8 pagesRapid Crash Course: Single CorrectHudsun HornetNo ratings yet
- Molecular RearrangementsDocument29 pagesMolecular RearrangementsThabiso GwijiNo ratings yet
- Sample Acs Final ExamDocument27 pagesSample Acs Final Examjilo100% (2)
- 2B-Alcohols, Ethers & Phenols - FINAL - 06!03!14 (86-112)Document27 pages2B-Alcohols, Ethers & Phenols - FINAL - 06!03!14 (86-112)udaysrinivasNo ratings yet
- CHM 2201 - Tutorial # 7-2017Document2 pagesCHM 2201 - Tutorial # 7-2017antonio latenNo ratings yet
- How To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inDocument2 pagesHow To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inmyiitchemistry81% (16)
- Exercise StereochemistryDocument4 pagesExercise StereochemistryPuvaneswary LoganathanNo ratings yet
- Iit Jam Chemistry Core2014Document8 pagesIit Jam Chemistry Core2014Mahendra GanuboyinaNo ratings yet
- Alcohol, Ether & Phenol: Chapter Practice ProblemsDocument6 pagesAlcohol, Ether & Phenol: Chapter Practice ProblemsSushank MishraNo ratings yet
- Organic Chemistry I: CH H C CH DDocument4 pagesOrganic Chemistry I: CH H C CH DSankar AdhikariNo ratings yet
- Benzene (B)Document17 pagesBenzene (B)Variganji Sumanth BabuNo ratings yet
- HW 03 On IUPAC NamingDocument1 pageHW 03 On IUPAC NamingEMERALDARCANISTNo ratings yet
- Alcohol, Ether & Phenol: Chapter Practice ProblemsDocument6 pagesAlcohol, Ether & Phenol: Chapter Practice ProblemsAtharva GanjuNo ratings yet
- PS2 Carboxylic Acids and DerivativesDocument2 pagesPS2 Carboxylic Acids and Derivativesscarllee rogerNo ratings yet
- A - 1 (Isomerism, Reaction Mechanism) - Question PaperDocument11 pagesA - 1 (Isomerism, Reaction Mechanism) - Question PaperSachin DedhiaNo ratings yet
- Neet Sample Paper: Max. Marks: 180 Duration: 3 HrsDocument38 pagesNeet Sample Paper: Max. Marks: 180 Duration: 3 HrsShiv soniNo ratings yet
- Class Test-6 - Carboxylic Acid - Amines JEE Adv - CC - AnsDocument6 pagesClass Test-6 - Carboxylic Acid - Amines JEE Adv - CC - Ansbruh pogNo ratings yet
- Revision Notes Organic ChemistryDocument30 pagesRevision Notes Organic ChemistryAtharav Porwal100% (1)
- Name Reactions Perkin Reaction DPPDocument1 pageName Reactions Perkin Reaction DPPSDMNo ratings yet
- EstersDocument1 pageEstersla physique selon le programme FrançaisNo ratings yet
- Basics of Photochemistry and Norrish Type I Reaction: Presented By: Dr. Nidhi VashisthaDocument12 pagesBasics of Photochemistry and Norrish Type I Reaction: Presented By: Dr. Nidhi Vashisthanidhi vashisthaNo ratings yet
- Excel JEE Booster (3A, 3B) Chemistrty Alcohol Phenol and EtherDocument21 pagesExcel JEE Booster (3A, 3B) Chemistrty Alcohol Phenol and Ethersourav gargNo ratings yet
- Isomerism DPP - With Solution PDFDocument20 pagesIsomerism DPP - With Solution PDFPiyush Agarwal50% (2)
- Aromatic Compounds - QuestionDocument14 pagesAromatic Compounds - Questionhrishik guptaNo ratings yet
- CSIR Test Paper - 16Document32 pagesCSIR Test Paper - 16Vineeth V TNo ratings yet
- Exercise - III Subjective Level-I: CH-CHDocument3 pagesExercise - III Subjective Level-I: CH-CHPriyanshu RajNo ratings yet
- 01 02 2023 Chemistry - Paper+With+Answer - MorningDocument6 pages01 02 2023 Chemistry - Paper+With+Answer - MorningLanaNo ratings yet
- Acidic Strength Order - 240203 - 170550Document4 pagesAcidic Strength Order - 240203 - 170550DEV SHARMANo ratings yet
- Acidic Strength Order 240203 170550Document4 pagesAcidic Strength Order 240203 170550agroindustriesmeerutNo ratings yet
- CY2102Document2 pagesCY2102Prarabdha SharmaNo ratings yet
- Exam #2 PracticeDocument7 pagesExam #2 PracticeNiel Felix Villanueva BalakidNo ratings yet
- Module AG Sir - Organic Advanced - HydrocarbonsDocument16 pagesModule AG Sir - Organic Advanced - HydrocarbonsSai KrishnaNo ratings yet
- CMB - Mains - 16-07-2022 - After Corrections at 15.58 On 12-07-22Document5 pagesCMB - Mains - 16-07-2022 - After Corrections at 15.58 On 12-07-22Tanush AgarwalNo ratings yet
- Trabajo Quimica Superior NAVIDADDocument13 pagesTrabajo Quimica Superior NAVIDADSebastian GuerraNo ratings yet
- NJ Sir DPP PDFDocument899 pagesNJ Sir DPP PDFPiyush Dubey100% (1)
- Carboxylic Acid & Derivatives-02 - Solved ProblemsDocument15 pagesCarboxylic Acid & Derivatives-02 - Solved ProblemsRaju SinghNo ratings yet
- Exercise - V (Matrix) : O OH H CH OhDocument2 pagesExercise - V (Matrix) : O OH H CH OhAnant Preet SinghNo ratings yet
- VITEEE Chemistry 2013: - Download FromDocument11 pagesVITEEE Chemistry 2013: - Download FromAnweshMishraNo ratings yet
- A) OH B) OH C) OH D) OH E) OH F) OHDocument4 pagesA) OH B) OH C) OH D) OH E) OH F) OHRodrigo RVNo ratings yet
- A Graduate Course in NMR Spectroscopy (Ramakrishna V. Hosur, Veera Mohana Rao Kakita)Document321 pagesA Graduate Course in NMR Spectroscopy (Ramakrishna V. Hosur, Veera Mohana Rao Kakita)Amiteshwar SinghNo ratings yet
- Assignment 3Document2 pagesAssignment 3Jaykishan BosamiyaNo ratings yet
- Quantum Mechanics: Dr. B. M. Krishna MariserlaDocument12 pagesQuantum Mechanics: Dr. B. M. Krishna MariserlaGulzaar ChanniwalaNo ratings yet
- CollisionsDocument35 pagesCollisionsRaz MansorNo ratings yet
- 01 Physics 9 Chap 1Document47 pages01 Physics 9 Chap 1Ashraf Javed50% (2)
- Full Test-1 (Jee Main-2022) 28-03-2022 (f22 Hyd-Seniors) QPDocument13 pagesFull Test-1 (Jee Main-2022) 28-03-2022 (f22 Hyd-Seniors) QPsunny meenuNo ratings yet
- Gear QualityDocument3 pagesGear QualityMahender KumarNo ratings yet
- Consolidation NOTESDocument40 pagesConsolidation NOTESKarthi KeyanNo ratings yet
- 1 18 Atomic Physics The Nuclear AtomDocument18 pages1 18 Atomic Physics The Nuclear AtomRaheem Abdul ManyambaNo ratings yet
- Plane MirrorsDocument4 pagesPlane Mirrorskeyur.galaNo ratings yet
- Effects of Metakaolin On Autogenous Shrinkage of Cement PastesDocument8 pagesEffects of Metakaolin On Autogenous Shrinkage of Cement PastesAnonymous KEU60hNo ratings yet
- Mathematics For ElectromagnetismDocument20 pagesMathematics For ElectromagnetismPradeep RajasekeranNo ratings yet
- Spur Gears Component GeneratorDocument4 pagesSpur Gears Component GeneratorAndra KusumaNo ratings yet
- MiCOM P122 & P123Document3 pagesMiCOM P122 & P123Hari Krishna.MNo ratings yet
- Presentation - Atomic Structure and Mass SpecDocument23 pagesPresentation - Atomic Structure and Mass SpecDBXGAMINGNo ratings yet
- Solids Liquids SolutionsDocument18 pagesSolids Liquids SolutionsZaheer MohiuddinNo ratings yet
- Quiz4 SolDocument2 pagesQuiz4 SolManoj KumarNo ratings yet
- Fentanyl CitrateDocument2 pagesFentanyl CitrateMulayam Singh YadavNo ratings yet
- E MC2Document12 pagesE MC2jiljil1980100% (2)
- 1 IntroductoryDocument45 pages1 IntroductoryTuhin Sahu100% (1)
- IAL Physics Unit 1 MCQ QP PDFDocument49 pagesIAL Physics Unit 1 MCQ QP PDFMir Faiyaz HossainNo ratings yet
- SWOT Analysis Applied To Hybrid PDFDocument318 pagesSWOT Analysis Applied To Hybrid PDFCarlos Alberto de MendonçaNo ratings yet
- CMO 29 s2007 - Annex III BSCE Course SpecsDocument74 pagesCMO 29 s2007 - Annex III BSCE Course SpecsHenzkel CanoyNo ratings yet
- 09 Sysnoise TutorialDocument58 pages09 Sysnoise TutorialYogesh KorulkarNo ratings yet
- Radiation Fallout-GuamDocument24 pagesRadiation Fallout-GuamTroy LivingstonNo ratings yet
- Differential Equations If Matlab Code: PendulumDocument8 pagesDifferential Equations If Matlab Code: PendulumRaaj ChatterjeeNo ratings yet
- CadDocument8 pagesCadRamesh Babu GarlapatiNo ratings yet
- Trigonometry - Trigonometric Angles and Ratios Worksheet PDFDocument4 pagesTrigonometry - Trigonometric Angles and Ratios Worksheet PDFFons Roxas-ChuaNo ratings yet
- 3 Echoes in The Quantum SymphonyDocument2 pages3 Echoes in The Quantum SymphonyMarsuki HardjoNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (1)
- ICH Quality Guidelines: An Implementation GuideFrom EverandICH Quality Guidelines: An Implementation GuideAndrew TeasdaleNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- Essential Chemistry for Formulators of Semisolid and Liquid DosagesFrom EverandEssential Chemistry for Formulators of Semisolid and Liquid DosagesRating: 5 out of 5 stars5/5 (2)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Practical Approaches to Method Validation and Essential Instrument QualificationFrom EverandPractical Approaches to Method Validation and Essential Instrument QualificationNo ratings yet
- The Nature of Drugs Vol. 2: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 2: History, Pharmacology, and Social ImpactNo ratings yet
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeFrom EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNo ratings yet
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Dust Explosion and Fire Prevention Handbook: A Guide to Good Industry PracticesFrom EverandDust Explosion and Fire Prevention Handbook: A Guide to Good Industry PracticesNo ratings yet
- Guidelines for Integrating Process Safety into Engineering ProjectsFrom EverandGuidelines for Integrating Process Safety into Engineering ProjectsNo ratings yet
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (4)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Lime and Limestone: Chemistry and Technology, Production and UsesFrom EverandLime and Limestone: Chemistry and Technology, Production and UsesRating: 4 out of 5 stars4/5 (1)