Professional Documents
Culture Documents
Roselyn 1
Uploaded by
Lecsos clyde Pang-ag0 ratings0% found this document useful (0 votes)
15 views5 pagesOriginal Title
Roselyn1
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
15 views5 pagesRoselyn 1
Uploaded by
Lecsos clyde Pang-agCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 5
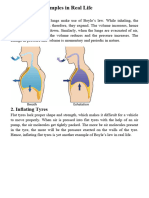
The working of a syringe can also be explained using
Boyle's Law. When the plunger of a syringe is pulled out,
the volume inside the barrel increases, resulting in a
decrease in the pressure inside the barrel. Fluids (such as
water) flow from a high-pressure area to a low-pressure
area.
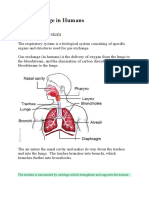
The operation of your lungs also can be explained using
Boyle’s Law. When you inhale (breathe in),
your diaphragm (a large muscle below your lungs)
lowers, which increases the volume inside your lungs.
This makes the air pressure inside your lungs lower than
the air pressure outside your lungs (and your body);
therefore, the outside air is drawn into your lungs (much
like the syringe). When you exhale (breathe out), your
diaphragm pushes upwards, reducing the volume inside
your lungs, increasing the pressure and forcing the air
outwards.
A
soda bottle, filled with a
mixture of carbon-di-oxide and water, is one of the best
examples to demonstrate Boyle's law. When the soda can
or bottle is sealed, it is difficult to compress. This is
because the air molecules present inside the container are
tightly packed and do not have space to move.
You can observe a real-life application of Boyle's Law
when you fill your bike tires with air. When you pump air
into a tire, the gas molecules inside the tire get
compressed and packed closer together. This increases the
pressure of the gas, and it starts to push against the walls
of the tire.
Spray paints work on the basis of Boyle's law. A
significant amount of pressure is exerted by the paint
molecules on the body of the can in which it is contained.
When the top of the can is pressed, the volume inside the
can gets reduced and the paint is thrown out with great
pressure.
You might also like
- Build This Bong: Instructions and Diagrams for 40 Bongs, Pipes, and HookahsFrom EverandBuild This Bong: Instructions and Diagrams for 40 Bongs, Pipes, and HookahsRating: 5 out of 5 stars5/5 (5)
- Applications of Gas LawsDocument3 pagesApplications of Gas LawsCharm GorospeNo ratings yet
- Applications of Boyle's LaawDocument2 pagesApplications of Boyle's Laawcherrymaeregalario2001No ratings yet
- BoyleDocument1 pageBoylePASTEURSYNCH GELBOLINGO, ERNIE IINo ratings yet
- LoveubabykoDocument2 pagesLoveubabykoSheila Mae NimNo ratings yet
- Why Do Gas Bubbles Get Larger As They Rise?: Why Does The Bubble Grow?Document4 pagesWhy Do Gas Bubbles Get Larger As They Rise?: Why Does The Bubble Grow?Princes Katherine VergaraNo ratings yet
- Soda BottleDocument3 pagesSoda BottleBenedick CruzNo ratings yet
- Boyles LawDocument2 pagesBoyles LawjaNo ratings yet
- Boyles LawDocument6 pagesBoyles LawMara TillesNo ratings yet
- Boyles LawDocument1 pageBoyles LawRevilloNo ratings yet
- ExamplesDocument12 pagesExamplesMoldir SerikNo ratings yet
- Boyle's LawDocument24 pagesBoyle's LawRalph Jasil Esparagoza EscanillasNo ratings yet
- Boyle's LawDocument10 pagesBoyle's Lawja.abarcaa15No ratings yet
- Uses and Applications of The Different Gas LawsDocument8 pagesUses and Applications of The Different Gas LawsWin7Addict100% (30)
- Cloud in A Bottle ExperimentDocument2 pagesCloud in A Bottle ExperimentKristal Jade ConstantinoNo ratings yet
- 4th Quarter Module 1Document2 pages4th Quarter Module 1jackielou trongcosoNo ratings yet
- Siyensikula-Boyle's LawDocument3 pagesSiyensikula-Boyle's Lawrolando dumlaoNo ratings yet
- LawDocument6 pagesLawJaymart BobilesNo ratings yet
- Drinking Straw: Crushing Can ExperimentDocument1 pageDrinking Straw: Crushing Can ExperimentvedasewahNo ratings yet
- Cloud in A BottleDocument3 pagesCloud in A BottleAditya GaurNo ratings yet
- Repiration Humans Info and Lung Experiment ExplainedDocument15 pagesRepiration Humans Info and Lung Experiment Explainedsusan nobregaNo ratings yet
- Balloon Lung ExperimentDocument4 pagesBalloon Lung ExperimentdyanenfalhadapNo ratings yet
- Science ScriptDocument2 pagesScience ScriptTrixie San DiegoNo ratings yet
- Different Laws of Chemistry and Their Day ToDocument40 pagesDifferent Laws of Chemistry and Their Day ToSahejNo ratings yet
- Gas Laws Form 4 Exercise and ExamplesDocument6 pagesGas Laws Form 4 Exercise and ExampleszahidahoseinNo ratings yet
- Chapter 1: Respiration: ObjectiveDocument3 pagesChapter 1: Respiration: ObjectiveYugenNo ratings yet
- If You Play-WPS OfficeDocument1 pageIf You Play-WPS Officeawitg031No ratings yet
- De LEON, Cyrus Q. BSIE 1A (Cloud in A Bottle)Document2 pagesDe LEON, Cyrus Q. BSIE 1A (Cloud in A Bottle)Cyrus De LeonNo ratings yet
- Stemtokexperiment BoylelawDocument1 pageStemtokexperiment BoylelawAvegail AdoraNo ratings yet
- Definition of Boyle's Law: What Is An Ideal Gas?Document4 pagesDefinition of Boyle's Law: What Is An Ideal Gas?حسين كاظم ياسينNo ratings yet
- 3rd Activity Guide QuestionsDocument5 pages3rd Activity Guide QuestionsAcademic ServicesNo ratings yet
- SciDocument2 pagesSciErica BiancaNo ratings yet
- Inbound 2075202700053796033Document1 pageInbound 2075202700053796033geoffreypenaranda05No ratings yet
- Nota Air Pressure (Sains)Document7 pagesNota Air Pressure (Sains)Damairin SelangorNo ratings yet
- The Human Breathing System Part2Document23 pagesThe Human Breathing System Part2Delfin Jr UrbanoNo ratings yet
- The Science/Mechanics of BreathingDocument1 pageThe Science/Mechanics of BreathingVan MichaelNo ratings yet
- Properties of AirDocument6 pagesProperties of AirDaoud KhanNo ratings yet
- Breathing - and - Gas Exchange Igcse BiologyDocument88 pagesBreathing - and - Gas Exchange Igcse BiologyEllie HousenNo ratings yet
- Booboo Bubble ExperimentDocument4 pagesBooboo Bubble ExperimentmmmmmmmmmmmmmmmNo ratings yet
- Gas Exchange in HumansDocument5 pagesGas Exchange in HumansRAGHU RAM SAKAMURINo ratings yet
- Gideon Elisha A. Ballesteros 10-WisdomDocument1 pageGideon Elisha A. Ballesteros 10-WisdomRandomStuffNo ratings yet
- 3 Applications of Boyle S, Charle S and Gay-Lussac S LawDocument7 pages3 Applications of Boyle S, Charle S and Gay-Lussac S LawJeric MaribaoNo ratings yet
- G10 Science Q4 - Boyle's Law (Gas Law)Document13 pagesG10 Science Q4 - Boyle's Law (Gas Law)Sky HadesNo ratings yet
- Document For Activity 2.Document2 pagesDocument For Activity 2.Jhom Angelo BringasNo ratings yet
- Applications For Ideal Gas LawsDocument6 pagesApplications For Ideal Gas Lawsdreamxtreme16No ratings yet
- BehaviorofGases3 3Document7 pagesBehaviorofGases3 3Melanie TrinidadNo ratings yet
- Chem Law LawDocument2 pagesChem Law LawFaye Lianne MaderoNo ratings yet
- The Action of Diaphragm in The Breathing MechanismDocument15 pagesThe Action of Diaphragm in The Breathing MechanismIbrahim AhmadNo ratings yet
- Features of Gas Exchange Surfaces: The Alveolus Is The Gas Exchange Surface in HumansDocument9 pagesFeatures of Gas Exchange Surfaces: The Alveolus Is The Gas Exchange Surface in HumansM. ShayanNo ratings yet
- Gas LawsDocument20 pagesGas Lawsapi-305343335No ratings yet
- DocumentDocument1 pageDocumentcristinemaelumanao18No ratings yet
- Understanding BernoulliDocument2 pagesUnderstanding BernoulliArul AnanthanNo ratings yet
- 11.1 The Breathing System: Features of Gas Exchange SurfacesDocument13 pages11.1 The Breathing System: Features of Gas Exchange SurfacesX GamerNo ratings yet
- Air PressureDocument21 pagesAir PressureNoredah JamiaanNo ratings yet
- How Does A Siphon WorkDocument22 pagesHow Does A Siphon WorkVinothNo ratings yet
- Charles LawDocument12 pagesCharles LawLerr Real RelleNo ratings yet
- SciDocument1 pageSciJasmine BarquillaNo ratings yet
- Boyles Law CompleteDocument4 pagesBoyles Law CompleteLincoln PiaoanNo ratings yet
- Script 1Document1 pageScript 1hUnTer X gamingNo ratings yet
- (ALBN) FizziologyDocument4 pages(ALBN) FizziologyClaudemir HackerNo ratings yet