Professional Documents
Culture Documents
Air Pollution and Ozone
Uploaded by
Wajid DaurCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Air Pollution and Ozone
Uploaded by
Wajid DaurCopyright:
Available Formats
Environmental Science
Environmental Pollution
Unfavorable alterations in our surroundings are sources of environmental pollution.
Air Pollution
The contamination of air with dust, smoke, smog, harmful gases and other harmful substances
which lead adverse effects on life and quality of life is called air pollution.
Air Pollutant: agents which cause air pollution are called air pollutants. Air pollutants affect the
living things and disturbs the ecosystem. There are two types of air pollutants:
i. Primary pollutants:
The waste products of the industries and the exhaust of automobiles which are directly
added to the environment are called primary pollutants e.g. SO2, SO3, NO2, NO, CO, HC
(methane), PM, NH3, CFC, VOC, Radioactive substances etc.
ii. Secondary pollutants:
The primary pollutants undergo many reactions in the atmosphere and produce new
pollutants called secondary pollutants e.g. H2SO4, HNO3, H2CO3, HF, Ozone,
Peroxybenzol, PAN, Aldehyde, Ketone etc.
Air Quality Index (AQI)
An Air Quality Index (AQI) is a number used by government agencies to measure the air
pollution levels and communicate it to the population. Air pollution in Lahore, Pakistan is on the
rise once again. Lahore declared world’s most polluted city after Delhi in 2022. Since last two
years 2021 & 2022, Lahore topped the list of Air Quality in the world with hazardous AQI (Air
Quality Index) levels going as high as approximately 397.
Dr. Waheed Mushtaq
Environmental Science
Emission Sources of Air Pollution
• 52% industry
• 27% Transportation
• 8% commercial & consumer products
• 10% agriculture
• 1% residential heating
• 2% others
Air Pollution Control Equipment
Control devices for particulate contaminants Control devices for gaseous contaminants
i. Gravitational settling i. Wet Absorption Method
ii. Cyclonic separators ii. Dry Absorption Method
iii. Fabric filters iii. Chemical treatment
iv. Electrostatic precipitators (Reduction or conversion)
v. Scrubbers (wet collectors) iv. Green Chemistry
Air pollutants, their sources and health effects
AIR POLLUTANTS SOURCES HEALTH EFFECTS
Oxides of Nitrogen Bacterial action Acid rain
(NOx) (especially NO)
Power plants Lungs irritation
Oil refineries
Fossil Fuel burning
Respiratory infections and asthma
From industries
Automobiles
(Internal combustion of Cardiovascular disease
engine)
Pulmonary edema (too much fluid in lungs)
Oxides of Sulphur Volcanic eruption (67%) Pungent odour, very irritant and
(SO2 or SO3 called Coal burning suffocating
as Sox) Combustion of fossil Irritation to eyes, nose & throat
fuels
Dr. Waheed Mushtaq
Environmental Science
Oxidation of sulphur Respiratory problems (Asthma)
Petroleum industries Heart diseases
Brain, liver damage
Acid rain
Carbon monoxide Volcanic eruption Highly poisonous and causes suffocation
Oxidation of methane because of blockage of haemoglobin for
Fuel burning (75% CO) oxygen.
Combustion of fossil
High concentration cause Oxygen
fuels
From industries starvation (Anoxia) with symptoms of
headache, fatigue (temporary loss of
strength and energy), nausea, chest pain
and unconsciousness.
Eventually death if exposure is for longer
time.
Volatile organic Automobiles VOC are carcinogens
compound Industrial Processes Eyes, skin irritation
(Benzene, Toluene, Headache, nausea
(VOC) ethylene glycol, xylene, Damage to liver, kidney, CNS etc
chloroform etc)
Combustion of fossil
fuels
Peroxyacetyl nitrate Plastic burning Eyes, skin irritation
(PAN) & Industrial processes Headache, nausea
Tropospheric Ozone Lungs inflammation
Asthma
Heavy metals Batteries Skin & lungs cancer
(Pb, Cr, Hg etc) Paints Gastrointestinal and kidney dysfunction
Vehicular emission Birth defects
(TEL)
Radon gas Radioactive decay of Damage lung tissue and lead to lung cancer
uranium produce
Rn 222 & radiogenic noble gases
Rn-222 (thoron) (Rn & thoron)
Particulate matter Sea salt, dust, Pollen PM10 effect eye, nose, throat
Microscopic Aerosol PM5 throat infection
suspended particles Burning of form lands PM2.5 lungs damage such as
(solids or liquid Natural gaseous Asbestosis: white lung disease
droplets) in the air. precursors Silicosis: Black lung disease
Size measured in um
Dioxins By products of industrial Cause cancer
practices. Reproductive and developmental problems
Chlorinated
Incineration processes Damage to the immune system
dibenzo-p-dioxins
Dr. Waheed Mushtaq
Environmental Science
(CDDs) Forest fires Interfere with hormones.
Volcanoes
Polychlorinated
biphenyls (PCBs)
OZONE DEPLETION
Decrease in the thickness of ozone layer in stratosphere due to chlorofluoro carbons (CFCs) and
other refrigerating agents is called ozone depletion.
Ozone (O3) is a pale blue gas and is an allotrope of oxygen present in both troposphere and
stratosphere. The ozone layer in the troposphere is pollutant because it causes irritation of the
eyes but the ozone layer in the stratosphere is not pollutant because it protects us from the U.V.
rays (especially UV-B & UV-C radiation having wavelength 285-315nm) of the sun and acts as
umbrella of earth. Ozone layer is located approximately 20 to 30 km above earth.
Ozone hole: The thickness of the ozone layer generally varies with seasons and other
geographical factors. However, average ozone layer thickness in stratosphere is 300 DU or 3mm
(1 Dobson’s Unit=0.01mm) above a height of 20 to 30 km from earth. The region in ozonosphere
where the concentration of ozone is less than 220 DU is called ozone hole. Ozone depletion is
mainly observed in every year during Sep – Nov. In the scientific journal Nature on May 16,
1985, three scientists from the British Antarctic Survey announce their detection of abnormally
low levels of ozone over the South Pole. In Antarctic region, significant ozone depletion rate is
due to formation of polar stratospheric clouds (PSCs).
Sources of Ozone depleting substances (ODSs)
Referigerating agents
Aerosol spray
Foam making
Organic synthesis
Fire extinguishers
Name of Ozone depleting substances (ODSs)
Dr. Waheed Mushtaq
Environmental Science
Oxides of Nitrogen
Chlofluorocarbons (CFCs)
Hydrochlorofluorocarbons (HCFCs)
Halons
Nuclear tests
Methyl Chloride
Carbon tetrachloride
Carbon tetrafluoride
Oxides of Nitrogen (NOx)
Nitrogen oxdies especially nitric oxide (NO) break the ozone molecule in the atmosphere by
following reaction:
NO + O3 NO2 + O2
NO2 + O NO + O2
Chlorofluorocarbons (CFCs) & Hydrochlorofluorocarbons (HCFCs)
Chlorofluorocarbons (CFCs) & Hydrochlorofluorocarbons (HCFCs) are generally a mixture of
chemicals that are generally contain different substances, including fluorine, carbon, chlorine,
and hydrogen.
Freon is a generic descriptor for a number of halocarbon products such as
dichlorodifluoromethane-CF2Cl2 (designated "Freon-12", "R-12", or "CFC-12"),
Chlorodifluoromethane (R-22 or HCFC-22), Trichlorofluoromethane- CFCl3 "Freon-11". Freons
are significant refrigerant and aerosol spray propellants.
The reactions are as,
CFCl3 + UV light → °CFCl2 + °Cl
CF2Cl2 + UV light → °CF 2 Cl + °Cl
°Cl + O3 → °ClO + O2
°ClO + °O → °Cl + O2
One chlorine free radical can destroy up to 100,000 ozone molecules.
Dr. Waheed Mushtaq
Environmental Science
They are commonly used as a refrigerant, as propellants in aerosol sprays and in firefighting
agents.
Effects of Ozone depletion
Skin Cancer: (Melanoma & Non-melanoma)
Sunburn
Immunity system suppression
Eye cataract
Decrease in agriculture production
Decrease in forest productivity
Infertility of soil
Global warming
Acidic deposition
Photochemical smog
Degradation of outdoor paints & plastics
Genetic mutation
Disturbance of ecosystem
Global warming
In 1987, countries around the world came together to sign the Montreal Protocol and entered
into force on 1st January 1989, which formalized the mission to protect and repair the ozone layer
by rapidly reducing the volume of ozone depleting gases being released into the atmosphere. It's
the only UN treaty that has been ratified by all 198 UN member states.
Dr. Waheed Mushtaq
Environmental Science
Ozone Depleting Potential (ODP)
Ozone depleting potential is a measure of how much damage a chemical can cause to the
ozone layer compared with a similar mass of trichlorofluoromethane (CFC-11). CFC-11, with an
ozone depleting potential of 1.0, is used as the base figure for measuring ozone depleting
potential.
Chlorodifluoromethane (R-22), for example, has an ODP of 0.05. CFC 11, or R-11 has
the maximum potential amongst chlorocarbons because of the presence of three chlorine atoms
in the molecule.
SMOG
The combination of the smoke and fog is called smog.
Smog is a visible type of intense air pollution. It is a threat to human health and quality of
environment. Smog is formed by mixing air with pollutants and exhaust gases resulting from
human activities. It may contain:
NOx (Oxides of Nitrogen)
SOx (Oxides of Sulphur)
Ozone (O3)
Dr. Waheed Mushtaq
Environmental Science
PAN (Peroxyacetylnitrate (CH3C(O)OONO2): A unique property of PAN is that it
is very stable at cold temperatures and easily decomposes to release NOx at warm
temperatures.)
Soot particles (carbon black)
Hydrocarbons
Suspended particles
Particulate matter
TYPES OF SOMG
I. Reducing smog II. Oxidizing smog
I. Reducing smog
Composition: It contains high contents of SO2 and carbon soot particles
Sources: Burning of coal
Properties:
Blackish grey in colour
Also called Industrial Smog (as it is dominant in industrial sector)
Also called London Smog (as first time observed in London due to industrial
revolution in 19th century)
Reducing Smog (as Sulphur-dioxide (SO2) is a very good reducing agent as it can
easily gain electrons to oxidize itself and attain the oxidation state of +6 by
converting into SO3)
Winter Smog
Sulfurous Smog
Classical smog
Dr. Waheed Mushtaq
Environmental Science
II. Oxidizing Smog:
Composition: It contains NOx, PAN, O3, Hydrocarbons and VOC
Sources: Excessive vehicular emission
Properties:
Yellow Brownish in colour (due to NO2)
Also called Photochemical Smog (as it is produced due to photochemical
reactions). It is most common type of smog.
Los Angeles Smog (as first time observed in Los Angeles due to urbanization in
19th century)
Also called Urban Smog (as dominant in urban smog)
Also called Oxidizing Smog (As it consists of higher concentration of the
oxidants like O3 and NO2)
Summer Smog
Conditions for formation:
i. There must be sufficient NO, hydrocarbons and volatile organic compounds.
ii. Sunlight to speed up some reactions.
iii. The motion of air molecules should be small so that reactions are not disturbed.
iv. Humidity
v. Moderate temperature
Reactions involved in Smog formation
i. NO + VOC NO2
ii. NO2 + UV-light NO + °O
iii. O2 + °O O3
iv. NO2 + VOC PAN + Hydrocarbon etc.
NO + VOC + O2 + UV-light O3 + PAN + other oxidants
Harmful effects of smog
1. Coughing and wheezing
Dr. Waheed Mushtaq
Environmental Science
2. Birth defects
3. Burning sensation in eyes and throat
4. Risk of serious heart diseases
5. Risk of serious lung disease.
6. Dangerous for people suffering from asthma.
7. Smog can also kill plants.
8. PAN is eye irritant and also toxic for plants.
9. Premature death due to respiratory failure
10. Increase risk of Rickets (softening of bones in children)
11. Visibility level decrease
12. Road accidents
How to Control Smog
1. Using renewable sources of energy
2. Reducing the number of vehicles, and prefer to use public transport
3. Use of biodegrade able items that can be recycled
4. Use Smog towers, this has been used successfully in China.
Volcanic smog (known as vog) is a mixture of atmospheric gases, volcanic gases,
and suspended liquid and solid particles.
Acid rain:
A rain having a pH less than 5 and near to 4 is called an acid rain. Acid rain is a mixture
of sulphuric acid (H2SO4) and nitric acid (HNO3).
Common rain have pH approximately 5.6 to 6.0 and is slightly acidic due to presence of
carbonic acid (H2CO3).
Dr. Waheed Mushtaq
Environmental Science
Artificial Rain: Dry ice or solid carbon dioxide is used to create artificial rains. Artificial
rain is produced by spraying clouds with substances such as silver iodide (costly) or
cheaper ones such as solid carbon dioxide (dry ice).
Production:
It is formed due to the formation of acids sulphuric acid (H2SO4) and nitric acid (HNO3)
in the atmosphere.
2NO2 (g) + H2O (aq) → HNO3 (aq) + HNO2 (aq)
SO2 (g) + H2O (aq) + ½ O2 (g) → H2SO4 (aq)
Acidic deposition
Acid deposition, commonly known as acid rain, occurs when emissions from the
combustion of fossil fuels and other industrial processes undergo complex chemical
reactions in the atmosphere and fall to the earth as:
Wet deposition: Wet deposition is when the chemicals mix with rain, snow, cloud, fog
and descend to the ground.
Dry deposition: Dry deposition is when the chemicals become dry such as dry particles,
gas and descend to the ground. It can stick to buildings and cars.
Dr. Waheed Mushtaq
Environmental Science
Sources of Acid Rain
Anthropogenic sources:
Industrial emission (Textile, oil refineries)
Vehicular emission
Thermal power plants (burning of coal)
Burning of agriculture & domestic waste
Industrial sources:
Volcanic eruptions
Bacterial decomposition (Decomposition of dead plants & animals by bacteria)
Effects:
i. Acidification of soil which causes leaching of metals like Al, Hg, Pb and Ca into
the water bodies.
ii. Infertility of soil cause poor crop yield.
iii. Health hazards for the fish (Al clogging in fish gills)
Dr. Waheed Mushtaq
Environmental Science
iv. Health hazards for the fish eating birds
v. Health hazards for the fish eating human beings
vi. Biodiversity loss
vii. Deforestation
viii. Disturb aquatic ecosystem
ix. Damage terrestrial ecosystem
x. Acidification of soil leaches the nutrients and affects the growth of the plants
xi. It cause Marble Cancer (gradual deterioration of a monument's marble caused by
acid rain.). Such as White marble of Taj Mahal become yellowish due to near
located oil refineries. Other affected monuments are Colosseum of Rome, and the
Leaning Tower of Pisa.
CaCO3 + H2SO4 CaSO4 + H2CO3
xii. It increases process of corrosion of metals such as sculptural materials. The
statue of Liberty is made up of copper which reacts with acid rain and changes
its colour from red-brown to green.
xiii. Acidification of rocks cause their chemical weathering
xiv. It may enhance of iron rusting (Fe2O3. xH2O)
Dr. Waheed Mushtaq
You might also like
- Environmental Pollution-NOTESDocument41 pagesEnvironmental Pollution-NOTESMichael Scott100% (1)
- Brochure Rilsan-PA11 2005Document32 pagesBrochure Rilsan-PA11 2005idleffarzadsNo ratings yet
- Numerical ModelingDocument41 pagesNumerical Modelingntuten88No ratings yet
- Mathematics ContentDocument10 pagesMathematics ContentNayive Lancheros0% (3)
- Air Pollution: Patrick Ross S. DulayDocument31 pagesAir Pollution: Patrick Ross S. DulayPatrick Ross Serquiña DulayNo ratings yet
- Cblm-Smaw-Nc IiDocument80 pagesCblm-Smaw-Nc IiERIC NARAGANo ratings yet
- Biological Treatment of Palm Oil Mill Effluent (Pome) Using An Up-Flow Anaerobic SludgeDocument53 pagesBiological Treatment of Palm Oil Mill Effluent (Pome) Using An Up-Flow Anaerobic SludgeJim ChongNo ratings yet
- DHU-NOCL - JOB EXECUTION PLAN - SupersededDocument37 pagesDHU-NOCL - JOB EXECUTION PLAN - SupersededTaofiqNo ratings yet
- BGAS-MCQ-Exam QuestionsDocument11 pagesBGAS-MCQ-Exam QuestionsShanmuga Navaneethan100% (5)
- Air Pollution: by Bibhabasu MohantyDocument54 pagesAir Pollution: by Bibhabasu MohantyJaned PerwiraNo ratings yet
- Environmental Chemistry (Ustp)Document149 pagesEnvironmental Chemistry (Ustp)AnneNo ratings yet
- Lecture 6 Environmental-PollutionDocument71 pagesLecture 6 Environmental-PollutionD V MaskarNo ratings yet
- Environmental PollutionDocument66 pagesEnvironmental PollutionRAHUL JARARIYANo ratings yet
- 4158 Alia 1 Metode SamplingDocument71 pages4158 Alia 1 Metode SamplingoliviaNo ratings yet
- Env. PollutionDocument40 pagesEnv. Pollutionمریم رضویNo ratings yet
- Environmental Pollution (Final)Document25 pagesEnvironmental Pollution (Final)shreyaNo ratings yet
- Lecture 6 Environmental-Pollution-2 PDFDocument69 pagesLecture 6 Environmental-Pollution-2 PDFbholaNo ratings yet
- Air Pollution: CE1400 Environment and Safety Engineering Lecture-18Document32 pagesAir Pollution: CE1400 Environment and Safety Engineering Lecture-18Dinesh Kumar SahuNo ratings yet
- Air Quality Engineering 3Document77 pagesAir Quality Engineering 3Raven ReiiNo ratings yet
- Introduction To Air Pollution - 6 - 2021Document45 pagesIntroduction To Air Pollution - 6 - 2021Andreas KanimeNo ratings yet
- Env. PollutionDocument40 pagesEnv. PollutionRuqia ThaheemmNo ratings yet
- Introduction To Environment: Environment Is A Comprehensive Term Meaning Surroundings. It IncludesDocument15 pagesIntroduction To Environment: Environment Is A Comprehensive Term Meaning Surroundings. It IncludesUttam KonwarNo ratings yet
- Lec - Air Pollution Effects and ControlDocument40 pagesLec - Air Pollution Effects and ControlManya WadhwaniNo ratings yet
- Unit 5Document117 pagesUnit 5Gunjan MeenaNo ratings yet
- UNIT 4 Environmental Pollution NEW - Che110Document109 pagesUNIT 4 Environmental Pollution NEW - Che110Arihant Dev SharmaNo ratings yet
- By-Konark Prakash M.tech (Environmental Engineering)Document15 pagesBy-Konark Prakash M.tech (Environmental Engineering)TOSHIKA SONINo ratings yet
- Codeforcoder UNIT 4 PollutionDocument142 pagesCodeforcoder UNIT 4 PollutionYusuf Al NaiemNo ratings yet
- Air QualityDocument51 pagesAir QualitysNo ratings yet
- Air Pollution2Document31 pagesAir Pollution2daabgchiNo ratings yet
- Air Pollution: BY: John Roland Edaño Maria Theresa Aquino Camille Cabrera Noelle Catherine Diaz Danica SalorDocument37 pagesAir Pollution: BY: John Roland Edaño Maria Theresa Aquino Camille Cabrera Noelle Catherine Diaz Danica SalorMarvin MonterosoNo ratings yet
- Environmental Impacts of Energy SourcesDocument13 pagesEnvironmental Impacts of Energy SourcesSudip NeupaneNo ratings yet
- Air Pollution - Sources, Effects & ManagementDocument45 pagesAir Pollution - Sources, Effects & ManagementSrijaJuluruNo ratings yet
- PollutionDocument52 pagesPollutionSoham MukherjeeNo ratings yet
- Air PollutionDocument97 pagesAir PollutionPMNo ratings yet
- Environmental Pollution and ControlDocument56 pagesEnvironmental Pollution and ControlNazrul IzdhamNo ratings yet
- Air PollutionDocument31 pagesAir Pollutionapi-3734333No ratings yet
- Characterisation of Pollutants Sources of PollutantsDocument15 pagesCharacterisation of Pollutants Sources of PollutantsRohan ChauguleNo ratings yet
- Pollution and Its ControlDocument21 pagesPollution and Its ControlPriya TikadarNo ratings yet
- Air Pollution: By:-Anas Haruna IndabawaDocument20 pagesAir Pollution: By:-Anas Haruna IndabawaRenilda Dsilva100% (1)
- Module 6Document8 pagesModule 6Divine Grace CincoNo ratings yet
- Outdoor Air PollutionDocument38 pagesOutdoor Air PollutionDalia Talaat WehediNo ratings yet
- Presented by (Group 4) :-: Sumit Kumar, Pradeep Kumar, Sushant Daga, SourabhDocument13 pagesPresented by (Group 4) :-: Sumit Kumar, Pradeep Kumar, Sushant Daga, SourabhSourabh VeerwalNo ratings yet
- Lecture 5 & 6 (Ce-441) Air PollutionDocument31 pagesLecture 5 & 6 (Ce-441) Air Pollutionassad ullahNo ratings yet
- SCI1601Document102 pagesSCI1601maaahiiNo ratings yet
- Environmental Education Is Contextualizing Environmental Issues Within The Physical, Biological, SocialDocument3 pagesEnvironmental Education Is Contextualizing Environmental Issues Within The Physical, Biological, SocialClarisse QuintoNo ratings yet
- DR Adwitiya Das: Assistant Professor Department of Community Medicine Medical College Hospital, KolkataDocument18 pagesDR Adwitiya Das: Assistant Professor Department of Community Medicine Medical College Hospital, KolkataTirtharaj DebnathNo ratings yet
- Darshan PCSM Chapater 2 Air PollutionDocument31 pagesDarshan PCSM Chapater 2 Air PollutionHarsh PatelNo ratings yet
- ES Chapter 3Document31 pagesES Chapter 3patelbharatiben16No ratings yet
- Unit 2 (Pollution) Part 1Document53 pagesUnit 2 (Pollution) Part 1kumar.abhinav1015No ratings yet
- Environmental Science: Dr. Hemanta MedhiDocument16 pagesEnvironmental Science: Dr. Hemanta MedhiItmej NNo ratings yet
- Ce 112 Module 6 Environmental ChemistryDocument19 pagesCe 112 Module 6 Environmental ChemistryDana Clarisse M. GalutNo ratings yet
- Air PollutionDocument68 pagesAir PollutionjohnnyNo ratings yet
- Ilovepdf - Merged 1 1Document105 pagesIlovepdf - Merged 1 1pratik kmrNo ratings yet
- Environmental PollutionDocument54 pagesEnvironmental PollutionAman ShrivastavaNo ratings yet
- Lecture No.04 Env - SciDocument12 pagesLecture No.04 Env - SciZarmeen GulNo ratings yet
- Unit 4 L1 Pollution 05102020Document10 pagesUnit 4 L1 Pollution 05102020Vimal KunwarNo ratings yet
- Pollution Control - 1st - ChapterDocument30 pagesPollution Control - 1st - Chapterkivepe9583No ratings yet
- Ce 112 Module 6 Environmental ChemistryDocument19 pagesCe 112 Module 6 Environmental ChemistryAngelo GranadaNo ratings yet
- Chapter 11 - Part 2 - Air Pollution and Climate ChangeDocument41 pagesChapter 11 - Part 2 - Air Pollution and Climate ChangeAngel RabayaNo ratings yet
- Environmental Science and Engineering: 20BSCY201Document17 pagesEnvironmental Science and Engineering: 20BSCY201HARSHITHA M SEC 2020No ratings yet
- 5a AirPollution Material, TXTDocument7 pages5a AirPollution Material, TXTSoumya GhoshNo ratings yet
- Air PollutionDocument10 pagesAir PollutionAngelie LapeNo ratings yet
- Environmental Science Pollution and Its FactorsDocument16 pagesEnvironmental Science Pollution and Its FactorsShaira GadianoNo ratings yet
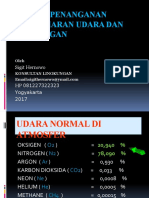
- Sesi 2..teknik Penanganan Pencemaran Udara Dan KebisinganDocument73 pagesSesi 2..teknik Penanganan Pencemaran Udara Dan KebisinganRezaNo ratings yet
- EVS PPT Air PollutionDocument27 pagesEVS PPT Air PollutionKajal SainiNo ratings yet
- Computer Physics Communications: Vei Wang, Nan Xu, Jin-Cheng Liu, Gang Tang, Wen-Tong GengDocument19 pagesComputer Physics Communications: Vei Wang, Nan Xu, Jin-Cheng Liu, Gang Tang, Wen-Tong Genglixiang RaoNo ratings yet
- MF 7009 - Non Destructive Evaluationmay June 2016Document2 pagesMF 7009 - Non Destructive Evaluationmay June 2016kannankrivNo ratings yet
- Kul 11 - Kebutuhan Energi Status GiziDocument35 pagesKul 11 - Kebutuhan Energi Status GiziAdelsy Dapa NgailoNo ratings yet
- Aurora EssayDocument2 pagesAurora EssayNovira ChandisaNo ratings yet
- 12th Grade Chemical Kinetics WorhshhetDocument1 page12th Grade Chemical Kinetics WorhshhetAmen RaipurNo ratings yet
- S&P Global Platts Global-Bunker-Fuels & IndicesDocument45 pagesS&P Global Platts Global-Bunker-Fuels & IndicescaptkcNo ratings yet
- Satmagan Description and II Info Oct 2005Document7 pagesSatmagan Description and II Info Oct 2005Ingridh D Quispe ChuanNo ratings yet
- Equipment Solutions.: Hp/HvofDocument12 pagesEquipment Solutions.: Hp/HvofSerhii MishchenkoNo ratings yet
- Synthesis of CamphorDocument1 pageSynthesis of CamphorangelofgloryNo ratings yet
- Two Solids Lab PDFDocument1 pageTwo Solids Lab PDFLukeNo ratings yet
- Substitution Elimination (Alkilhalides) Bab 5 FessendenDocument113 pagesSubstitution Elimination (Alkilhalides) Bab 5 Fessendenahmad jamalNo ratings yet
- Power Plant Inspection, Repair, and Testing: Learning ObjectivesDocument30 pagesPower Plant Inspection, Repair, and Testing: Learning ObjectivessamiNo ratings yet
- Bic Sodio Shandong MSDSDocument7 pagesBic Sodio Shandong MSDSFIORELLA VANESSA MAITA MUCHANo ratings yet
- techNOTE 11 - AR GlassfibresDocument3 pagestechNOTE 11 - AR GlassfibresJoseph JayakanthanNo ratings yet
- 3.0 Convection Heat Transfer-TeamsDocument83 pages3.0 Convection Heat Transfer-Teamsamira nabillaNo ratings yet
- Problem 14: Lead Iodide 1. The Graph Obtained Is One of Two Straight Lines, Meeting at A Peak of About 2.50 GDocument5 pagesProblem 14: Lead Iodide 1. The Graph Obtained Is One of Two Straight Lines, Meeting at A Peak of About 2.50 GLê Hoàng MinhNo ratings yet
- Metal Based PackagingDocument21 pagesMetal Based PackagingSheraz KarimNo ratings yet
- Effects of Parameter Changes On The Structure and Properties of Low-Density Polyethylene FoamDocument9 pagesEffects of Parameter Changes On The Structure and Properties of Low-Density Polyethylene FoamZunaida ZakariaNo ratings yet
- Micellar Catalysis or CatalystDocument39 pagesMicellar Catalysis or CatalystchinuasfaNo ratings yet
- Cambridge O Level: Physics 5054/11Document20 pagesCambridge O Level: Physics 5054/11iman jamilNo ratings yet
- 1-Handling of SolidsDocument10 pages1-Handling of SolidsAgus Wahyudhi100% (1)
- Ecosystem: Tiver &Document4 pagesEcosystem: Tiver &RUDRA PRATAPNo ratings yet
- MEEG 321 Homework 1 2018Document2 pagesMEEG 321 Homework 1 2018Abu Jafar RaselNo ratings yet